2025 AIChE Annual Meeting
(278a) Unraveling Lignin-Hemicellulose Interactions in Lignocellulosic Biomass: Insights from Acid-Catalyzed Pretreatment Using Quantitative Multi-CP Solid-State NMR
Authors
Lignocellulosic biomass is a promising feedstock to the production of sustainable fuels and chemicals. However, its complex and heterogeneous architecture, particularly the stable structure associated with lignin, hemicellulose, and cellulose, has significantly hampered the efficient of the biorefinery process. In particular, the molecular architecture of lignocellulose and its dynamic evolution during pretreatment are not yet fully understood, posing challenges in component separation and valorization. This study investigates the structural dissociation of lignin–hemicellulose networks and the associated lignin condensation behavior in the acid catalyzed biomass pretreatment process. We aim to develop a systematic and quantitative measure understanding how pretreatment influences the breakdown and reorganization of key molecular linkages. A state-of-art Multi Cross Polarization (Multi-CP) were applied with solid-state 13C Nuclear Magnetic Resonance (SS-NMR) spectroscopy techniques to monitor the structural changes of lignocellulosic biomass during at the molecular level, offering insights into the roles of specific lignin and hemicellulose subunits in biomass fractionation. The findings contribute to a deeper mechanistic understanding of lignocellulosic deconstruction and provide a conceptual basis for improving biomass fractionation strategies. This work supports the development of predictive tools to guide pretreatment optimization and enhance monomer yields in integrated biorefineries.
- Introduction:
Organosolv pretreatment technique is a widely recognized strategy to fractionate lignocellulosic biomass structure into the building block chemicals.1 In this process, acid dissociation mechanisms target specific hemicellulose bonds, such as glycosidic linkages which effectively facilitate the breakage of the complex plant cell wall matrix at reduced energy/chemical inputs.2 A clear molecular-level understanding of lignin–polysaccharide dissociation is essential for developing efficient pretreatment/fractionation strategies.
Hemicellulose is a particularly essential plant cell wall component to be dissociated in the pretreatment process. Hemicellulose is a branched polysaccharide structure linking the lignin and cellulose. It is composed of various components such as xylan. Briefly, Xylan is a major component of hemicellulose, consisting primarily of β-1,4-linked xylose units, and Xn2f and Xn3f are essential substructures of xylan. In this expression, "Xn" denotes xylans, while 2f and 3f indicate the specific configurations of the xylan backbone, such as the degree of branching or the presence of functional groups. These substructures can affect the interaction between hemicellulose and lignin, influencing the biomass's recalcitrance and the efficiency of pretreatment process. Arabinose (Ara) is a pentose which appears as side chains attached to the main xylan backbone, contributing to the branching and complexity of hemicellulose structures. Galactose (Gal) is a hexose present as part of galactoglucomannans or other polysaccharides. It can be involved in forming side chains or backbone structures, influencing the reactivity of hemicellulose. Understanding these substructures is crucial for optimizing biomass conversion, as they play significant roles in the integrity and chemical behavior of the plant cell wall structure.
The Multi-CP technique in SS-NMR is an advanced method designed to enhance signal sensitivity and resolution, particularly for complex samples like lignocellulosic biomass. Cross polarization is a method used to increase the signal intensity of nuclei with low natural abundance or low sensitivity, such as Carbon-13, by transferring polarization from nearby high-abundance nuclei, typically protons, through matching the Hartmann-Hahn condition. The Multi-CP technique applies sequential CPs for optimizing the polarization transfers for different carbons in the sample. This selective enhancement improves spectral resolution and sensitivity, allowing for better discrimination between various carbons, such as aromatic carbons in lignin or polysaccharides.3 The technique offers several advantages: it significantly boosts signal intensity and improves resolution. It is non-destructive, and facilitates quantitative analysis of the sample's composition. In the context of lignocellulosic biomass, Multi-CP is particularly useful for studying the structural dynamics and interactions between lignin and polysaccharides during pretreatment processes, providing insights into molecular architecture and changes at the molecular level, essential for optimizing biomass conversion strategies.
In this study, quantitative SS-NMR associated with Multi-CP technique was applied to investigate the changes of lignin and hemicellulose structure at carefully adjusted pretreatment conditions. Spectra peak deconvolution enabled quantification of the polysaccharides and evaluation of their hydrolysis kinetics. We discovered a strong positive correlation between the degradation of Xn3f with delignification efficiency, suggesting that specific hemicellulose motifs facilitate lignin release during pretreatment. These findings underscore the critical role of both spatial organization and chemical composition of hemicellulose in regulating lignin–polysaccharide dissociation.
- Experiments
Camellia Oleifera Oil Shell Biomass was treated using different oraganoslvs and acids under 66 sets of varied conditions to understand three major reactions, i.e. hemicellulose hydrolysis, lignin solubilization, and lignin re-condensation. After pretreatment, wet subtracted samples weighing 30–70 mg were filled into 3.2-mm zirconium rotors and analyzed using 400 MHz (9.4 Tesla) and 600 MHz (14.1 Tesla) Bruker Avance Neo spectrometers. The Multi-CP pulse sequence was applied to collect quantitative spectra under 15 kHz magic-angle spinning (MAS) at 296 K. Each experiment employed 7 CP blocks (1.1 ms contact time each), with a 0.9 s z-filter delay between blocks to allow for repolarization. The recycle delay was set as 2s. The 13C chemical shifts were calibrated by the adamantane CH2 peak at 38.48 ppm on the TMS scale. Spectrum deconvolution was carried out using the DMfit software. Hemicellulose substructures Xn2f, Xn3f, arabinose, galactose, and mannose were identified and quantified through spectral peak deconvolution.
To validate the NMR analysis, compositional analysis of the raw and pretreated biomass samples was performed according to the NREL protocol. In short, 150 mg of the samples were placed in 50 mL Falcon tubes and mixed with 1.5 mL of 72% sulfuric acid. The suspension was continuously stirred at 30°C for 1 hour, and then 42 mL distilled water was added to the solution and autoclaved at 121°C for 1 hour. The dissociated sugars were then determined using high-performance liquid chromatography (HPLC, Shimadzu). The remaining solids were oven-dried to determine the lignin content and placed in a furnace at 575°C for at least 6 hours to determine the ash content.
- Results and Discussion
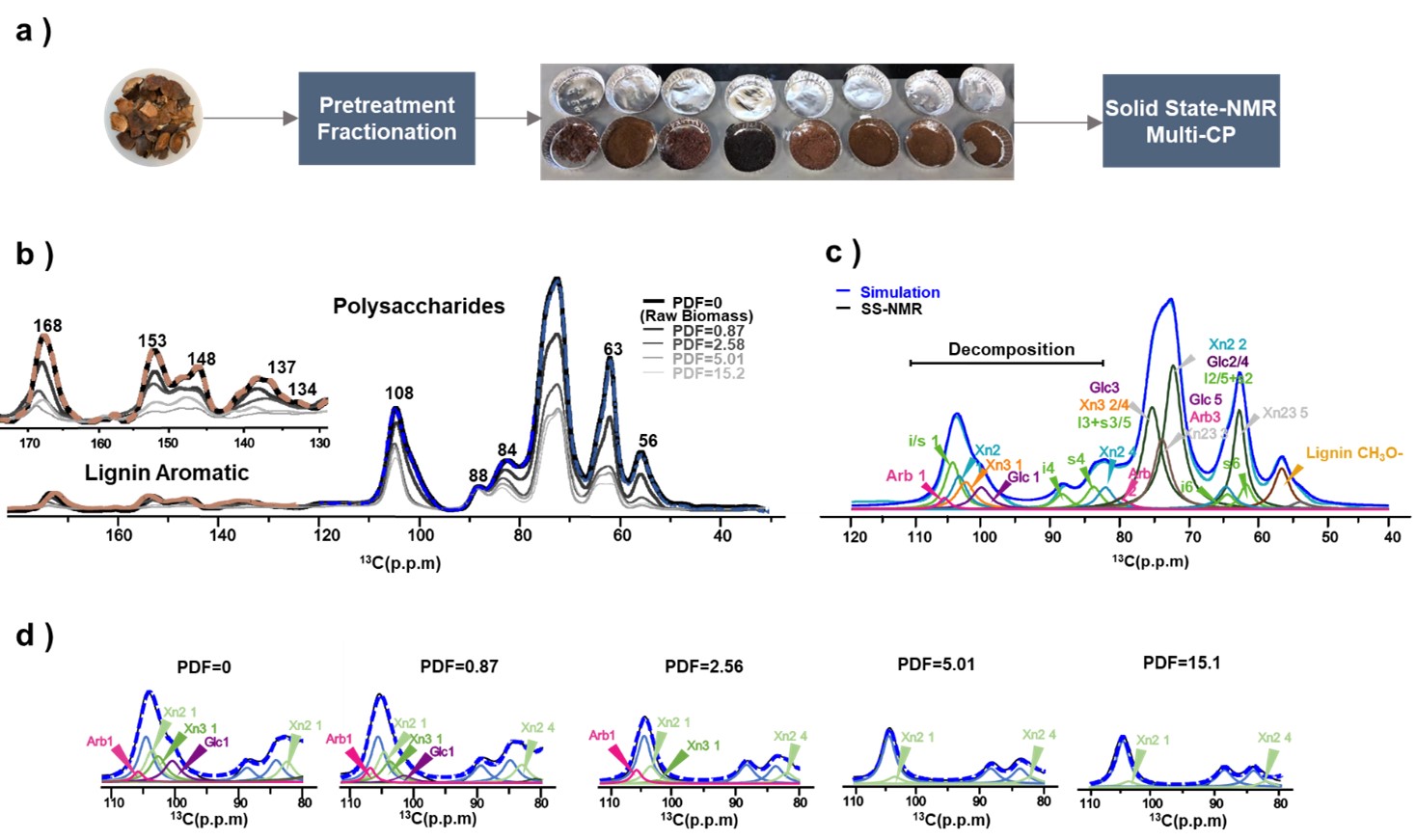
Solid-state 13C NMR with Multi-CP sequencing enabled the quantitative detection of aromatic and non-crystalline carbon structures in both native and pretreated biomass samples. Well-resolved spectra (Fig. 1b) revealed characteristic peaks for lignin aromatics (110–160 ppm) and hemicellulose C1 carbons (95–110 ppm). Peak intensities, normalized to the cellulose internal C4 signal at 88 ppm, declined progressively with increasing pretreatment severities (Fig. 1b). Although hemicellulose C2–C5 signals overlapped with cellulose in the 60–80 ppm region, spectral deconvolution (Fig. 1b&c) enabled accurate quantification of Ara, Gal, Xn2f, and Xn3f substructures. The deconvolution results closely matched with compositional analysis, confirming the method’s robustness without requiring destructive hydrolysis. Quantified NMR signals were used to calibrate a kinetic model describing the hydrolysis behaviour of individual hemicellulose components. Xn3f and Gal degraded significantly faster than Xn2f and Ara, consistent with their greater reactivity. These SS-NMR results provided not only compositional and spatial resolution but also the experimental basis for constructing a predictive model of hemicellulose degradation during organosolv pretreatment.
Figure 1. Solid-state 13C NMR spectra of COS biomass and lignin, highlighting key component and quantitative peak assignments.(a) Schematic workflow illustrating the biomass pretreatment fractionation process followed by solid-state MultiCP NMR acquisition; (b) One-dimensional 13C Multi-CP spectra of unlabeled COS cell walls before and after pretreatment;(c) Spectral deconvolution of the polysaccharide region, with peak assignments for Ara C1 (107.4 ppm), Xn2f C1 (103.8 ppm), Xn3f C1 (101.1 ppm), Glc C1 (100.8 ppm), and Xn2f C4 (82.0 ppm);(d) Comparison of deconvoluted spectra in the 80–115 ppm region across varying pretreatment severities, revealing changes in hemicellulose structure.