2025 AIChE Annual Meeting
(319c) Unlocking Hydrogen Carrier Potential: Integrated Mechanistic Studies and Surrogate Modeling
Authors
Building on these mechanistic insights, we extend our framework to compare hydride- and carrier-based strategies for hydrogen production, particularly focusing on electrochemical toluene hydrogenation. Using a bottom-up approach grounded in literature-derived Tafel data (activity, selectivity, and catalyst composition/loading), we employ a simplified (0D) electrolyzer model to identify governing parameters without immediate reliance on complex simulations. A subsequent reverse-design phase—coupled with surrogate modeling and targeted COMSOL-based sensitivity analyses—highlights key trade-offs between cost, activity, and stability, ultimately guiding the optimization of catalyst formulations. By integrating mechanistic, data-driven, and modeling perspectives, our work delivers actionable guidelines for enhancing catalyst performance and longevity across multiple hydrogen carrier pathways.
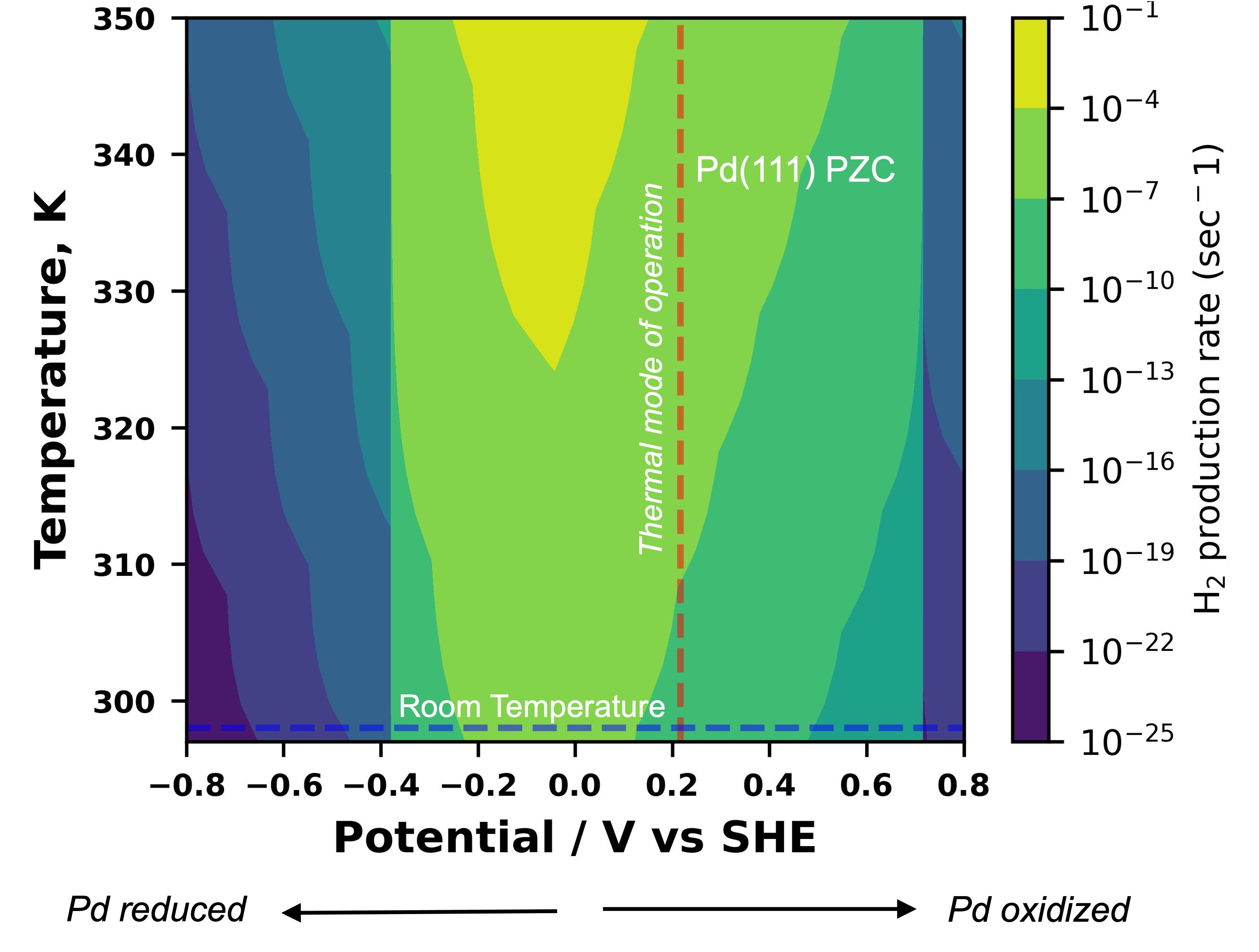
Figure 1. A heatmap for the variation of dehydrogenation rate (sec-1) against the operational choices of temperature and the electrode potential. the thermal mode of operation is denoted by the vertical red line (at the PZC of Pd), while the room temperature electrochemical route is shown via the horizontal blue line.