2025 AIChE Annual Meeting
(8b) Understanding (Stereo)-Selectivity in the (De)Hydrogenation of N-Heterocyclic Liquid Hydrogen Carriers over Metal Catalysts
Authors
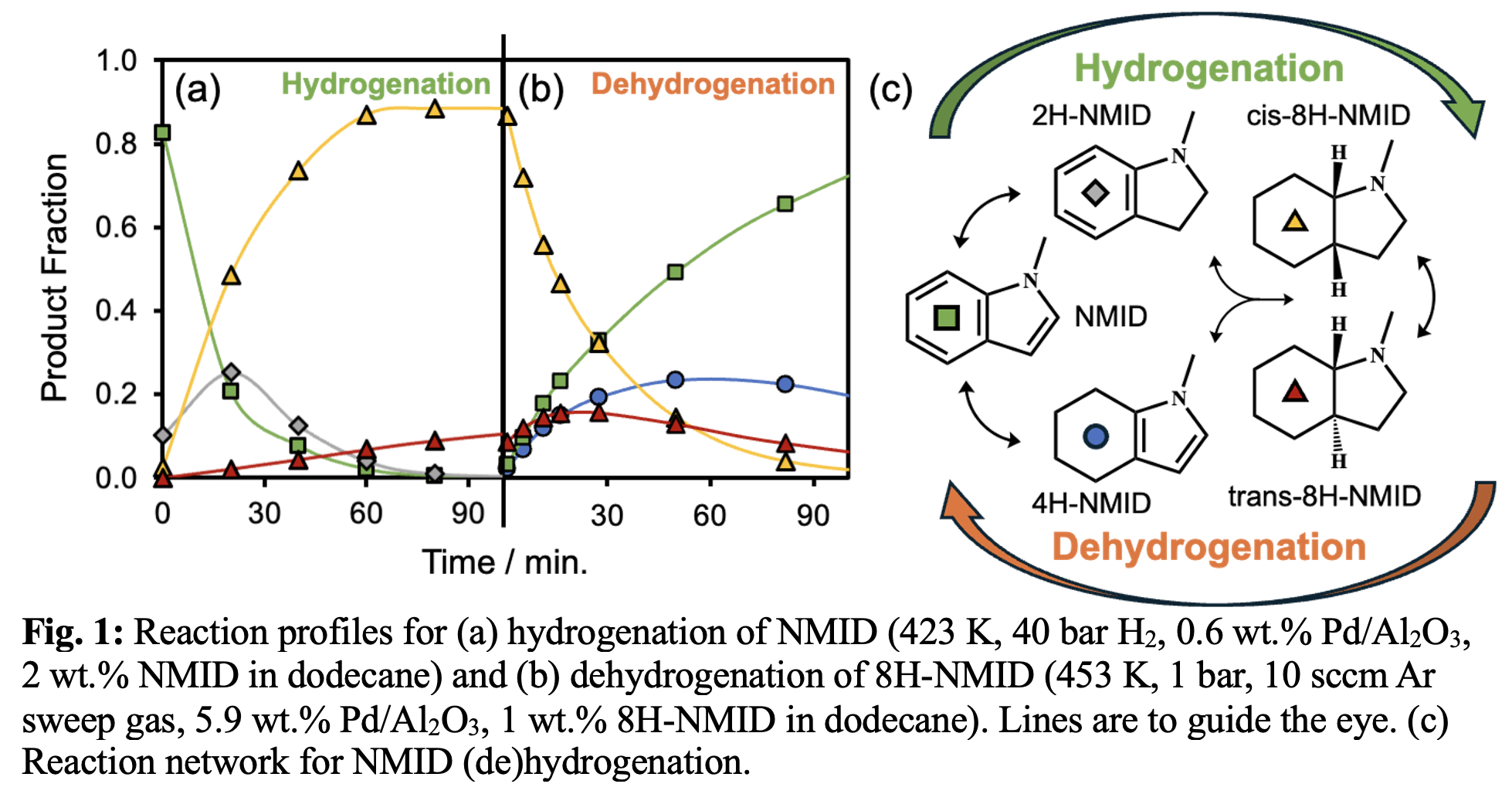
Here, we investigated chemo- and stereo-selectivity in the (de)hydrogenation of indole, N-methylindole (NMID), and 2-methylindole (2MID) over Pd/Al2O3 catalysts in (semi)-batch reactors. Studies showed C-H bond formation and breaking to be more facile in the less thermodynamically stable pyrrole ring: 2H-indole intermediates form via initial hydrogenation of the pyrrole ring; 4H-indole intermediates form via initial dehydrogenation of the pyrrolidine ring (Fig. 1c). While all (methyl)-indoles displayed similar hydrogenation intermediates, methyl groups on the pyrrole ring altered the kinetic stability of 2H-indole intermediates.
Monitoring stereochemistry provides unique mechanistic information about the direction of H atom attack to indole rings. Complete hydrogenation of indole led exclusively to the cis- isomer, consistent with syn facial addition of H atoms to planar-bound indole rings. In contrast, hydrogenation of NMID and 2MID lead to mixtures of cis- and trans- isomers. For NMID (Fig. 1a), a high cis/trans ratio (~20) is initially observed but decays towards an equilibrium ratio (~2) over at long reaction times, suggesting that the chiral C-H bonds are labile. Dehydrogenation of 8H-NMID reveals cis/trans isomerization which occurs in parallel with dehydrogenation steps (Fig. 1b); the cis- isomer has a 2x higher dehydrogenation rate constant than the trans- isomer based on reaction kinetics modeling. This work provides insights into the impacts of methyl group placement on the selectivity of N-heterocycle (de)hydrogenation reactions, informing the design of carriers and catalysts for hydrogen storage reactions.