2025 AIChE Annual Meeting
(118h) Understanding the Role of Cooperative Interactions in the Nitroaldol Reaction: Tuning Pore Diameter and Surface Density of Aminosilica Catalysts for Improved Activity
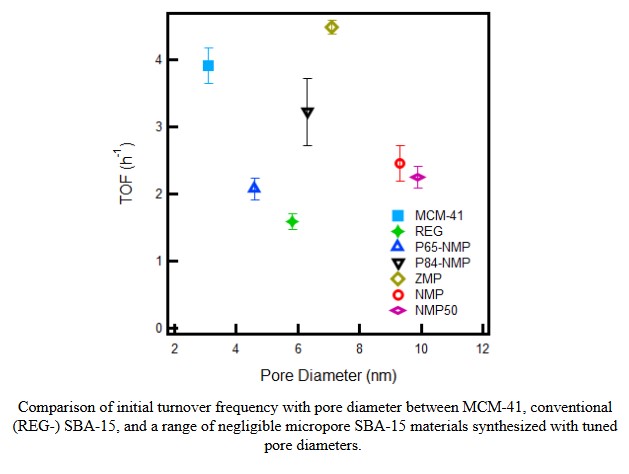
However, multiple layers of uncertainty remain in results of existing work that make the desired parameters for tuning unclear. Previous work shows a dependence of activity on pore diameter and suggests that cooperative interactions between basic amine sites and acidic surface silanols grow stronger under smaller confinement and promote greater activity in smaller pores. Conversely, it is reported that catalytic activity increases with surface density of amines on the support. Since high surface density decreases amine-silanol interaction, the influence of surface silanols and acid-base cooperativity on catalytic activity must be clarified.
In this work, we synthesize a novel line of SBA-15 supports with negligible microporosity and tuned pore diameter, which are grafted at similar surface densities. Analysis of activity reveals that materials with small pore sizes are not necessarily the most active and suggests that previously observed differences in activity are largely attributable to differences in microporosity between supports. Additionally, we confirm that activity increases with amine loading and investigate the cause, finding evidence that amine-silanol interactions inhibit activity for the nitroaldol reaction through solvent-induced stabilization of intermediates. Overall, this study advances material design by expanding the range of possibilities for SBA-15 synthesis, realizes high catalytic performance by tuning parameters of synthesis, and challenges the perceived role of acid-base interactions in nitroaldol chemistry.