2025 AIChE Annual Meeting
(460d) Understanding Rate Determining Steps Along Unique Branches of Multivalued Rates during Methane Oxidation
Authors
Austin Morales - Presenter, University of Houston
Olaf Deutschmann, Karlsruhe Institute of Technology (KIT)
Praveen Bollini, University of Houston
Michael Harold, University of Houston
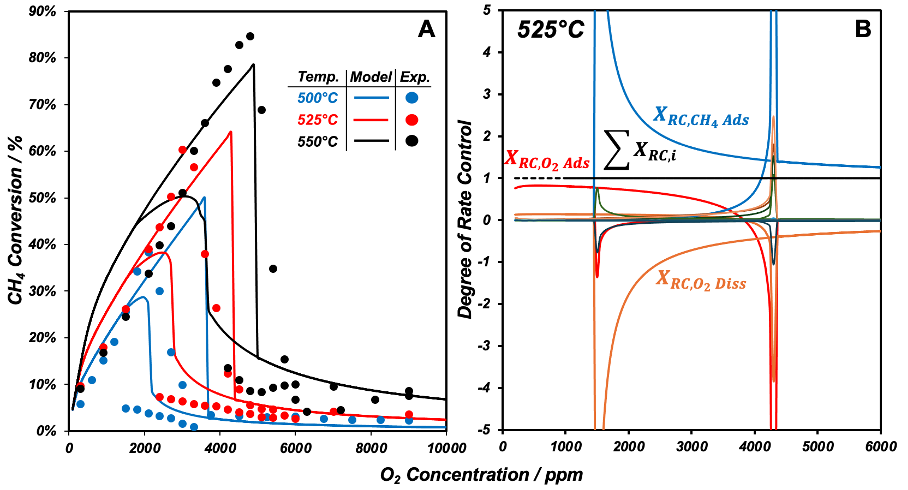
Natural gas (methane/ CH4) emissions are known to significantly accelerate global warming. Platinum (Pt) and palladium (Pd) catalysts with gaseous oxygen (O2) are commonly used to convert CH4 into less environmentally harmful CO2. Despite their effectiveness, competitive adsorption between O2 and CH4 along with their differing site requirements may induce isothermal multiplicity as illustrated in Fig. 1A. Experimentally measured and model estimated CH4 conversions at 525°C increase with O2 concentration forming an “ignited” branch reaching a maximum of 60% at 3500 (experimental) ppm O2. After which, the rate is extinguished to a lower branch with conversion of 8%. Decreasing the O2 concentration starting on the extinguished branch at 525°C (Fig. 1A) leads to an ignition in conversion at 2100 ppm O2 back onto the ignited branch. Unique solutions at equivalent experimental conditions beg the question as to which catalytic steps govern the individual branches. Mathematical formalisms including the degree of rate control can be computed along experimentally observed stable and unobserved unstable branches to elucidate their corresponding rate determining steps (RDSs). Fig. 1B shows that O2 adsorption has a degree of rate control near unity and is thus the RDS along the excited branch. Conversely, CH4 adsorption has the highest degree of rate control along the extinguished branch thereby denoting it the RDS. Degrees of rate control computed in Fig. 1B via a modified pseudo-arclength continuation algorithm demonstrate consistency as they sum to unity across all branches and coverages computed via site balances are equal to those estimated via thermodynamic degrees of rate control. This work not only identifies key RDSs along unique solutions of multivalued rates during catalytic methane oxidation but also demonstrates that degrees of rate control (and thus RDSs) are functions of both operating and initial conditions (path dependent).