2025 AIChE Annual Meeting
(309e) Understanding Molecular Processes That Control Selectivity in Organic Electrosynthesis
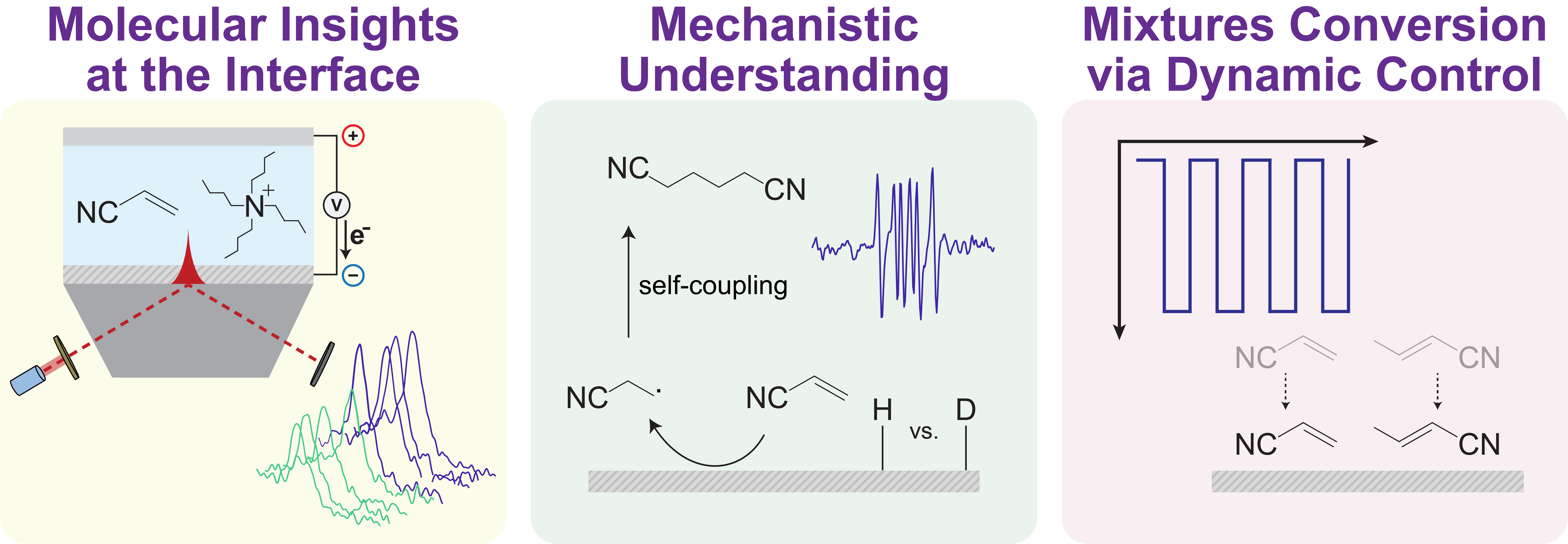
This work advances organic electrosynthesis through complementary approaches. First, we use in situ ATR-FTIR spectroscopy to show that tetraalkylammonium ions populate the electrical double layer, creating a microenvironment that favors interactions with organic molecules and enhances acrylonitrile concentration while expelling water molecules.9 Additionally, kinetic isotope effect studies reveal that propionitrile (PN) formation is rate-limited by proton transfer, while ADN formation likely is not. Electron paramagnetic resonance spectroscopy confirms the presence of free radicals during AN electroreduction, suggesting that coupling of PN radicals occurs primarily in the electrolyte. Finally, we demonstrate how electrochemical parameters governs product distributions in mixed-substrate electrosynthesis. Using high-throughput screening coupled with machine learning approaches, we systematically investigated the interplay between substrate composition, current density, and mass transport phenomena in the electrodimerization of acrylonitrile and crotononitrile mixtures. We reveal distinct reaction-limited and mass transport-limited regimes that dictate product selectivity, with preferential formation of adiponitrile occurring when radical generation from acrylonitrile outpaces that from crotononitrile under reaction-limited conditions. Furthermore, we demonstrate that pulsed electrolysis is a powerful technique for mixtures to precisely modulate the near-electrode microenvironment, enabling dynamic control over dimer distribution through strategic adjustment of active and rest periods. These findings establish a framework for understanding and controlling molecular processes at electrode interfaces in complex organic systems. The experimental techniques and reaction engineering strategies developed here open new possibilities for selective electrochemical transformations.
1. U.S. Department of Energy. Manufacturing Energy and Carbon Footprints Report. (2018).
2. Botte, G. G. Electrochemical manufacturing in the chemical industry. The Electrochemical Society Interface 23, 49 (2014).
3. Frontana-Uribe, B. A., Little, R. D., Ibanez, J. G., Palma, A. & Vasquez-Medrano, R. Organic electrosynthesis: a promising green methodology in organic chemistry. Green Chemistry 12, 2099-2119, doi:10.1039/c0gc00382d (2010).
4. Utley, J. Trends in organic electrosynthesis. Chemical Society Reviews 26, 157-167 (1997).
5. Moeller, K. D. Using Physical Organic Chemistry To Shape the Course of Electrochemical Reactions. Chem Rev 118, 4817-4833, doi:10.1021/acs.chemrev.7b00656 (2018).
6. McKenzie, E. C. R. et al. Versatile Tools for Understanding Electrosynthetic Mechanisms. Chem Rev 122, 3292-3335, doi:10.1021/acs.chemrev.1c00471 (2022).
7. Danly, D. Development and commercialization of the Monsanto electrochemical adiponitrile process. Journal of The Electrochemical Society 131, 435C (1984).
8. Seidler, J., Strugatchi, J., Gärtner, T. & Waldvogel, S. R. Does electrifying organic synthesis pay off? The energy efficiency of electro-organic conversions. MRS Energy & Sustainability 7, E42, doi:10.1557/mre.2020.42 (2021).
9. Mathison, R. et al. Molecular Processes That Control Organic Electrosynthesis in Near-Electrode Microenvironments. J Am Chem Soc 147, 4296-4307, doi:10.1021/jacs.4c14420 (2025).