2025 AIChE Annual Meeting
(114a) Understanding the Impact of Mass Transport on Electrochemical Carbon Dioxide Reduction Reaction
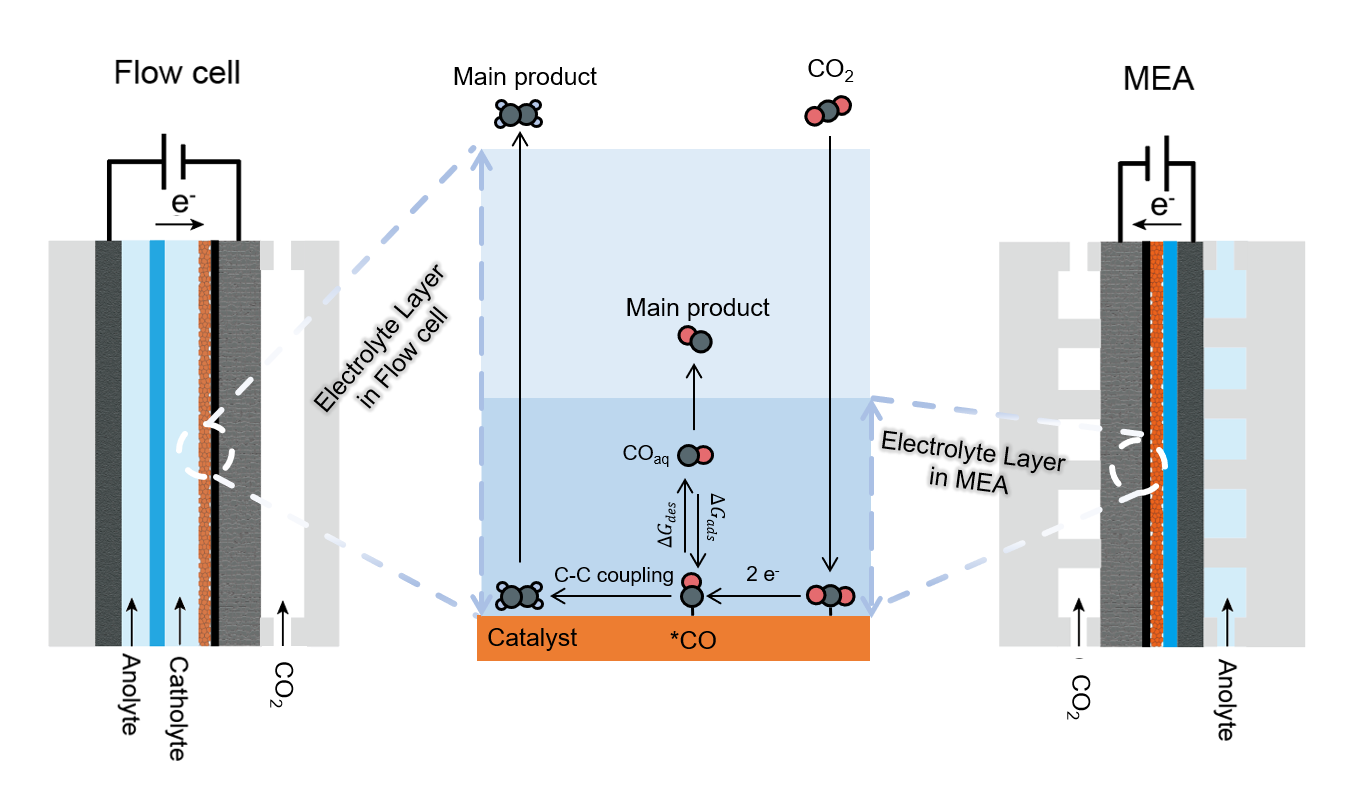
In this work, we discovered that flow cells exhibit high selectivity for ethylene, while MEAs show excellent selectivity for CO at low current density (< 200 mA cm-2) when Cu was used as the catalyst. Increasing the current density to 400 mA cm-2 will lead to an increase in Faraday efficiency of H2 in flow cells and ethylene in MEAs, highlighting the distinct microenvironment of catalysts in different configurations. The performance disparity between these two configurations largely diminished upon the introduction of a non-conductive protective layer in the flow cell. We proposed that the thickness of the electrolyte layer covering the catalyst, which corresponding to the CO2 diffusion length, is a crucial factor governing both CO2RR activity and selectivity. Furthermore, while improving CO2 mass transport did not necessarily enhance the selectivity of multi-carbon product, we found that it facilitated CO2RR under low CO2 partial pressures, offering a promising strategy for upgrading waste carbon emissions. These findings provide valuable insights into optimizing GDE structures and highlight the importance of mass transport in achieving efficient CO2 conversion.