2025 AIChE Annual Meeting
(551a) Understanding the Electronic Properties of Peptides and Peptoids
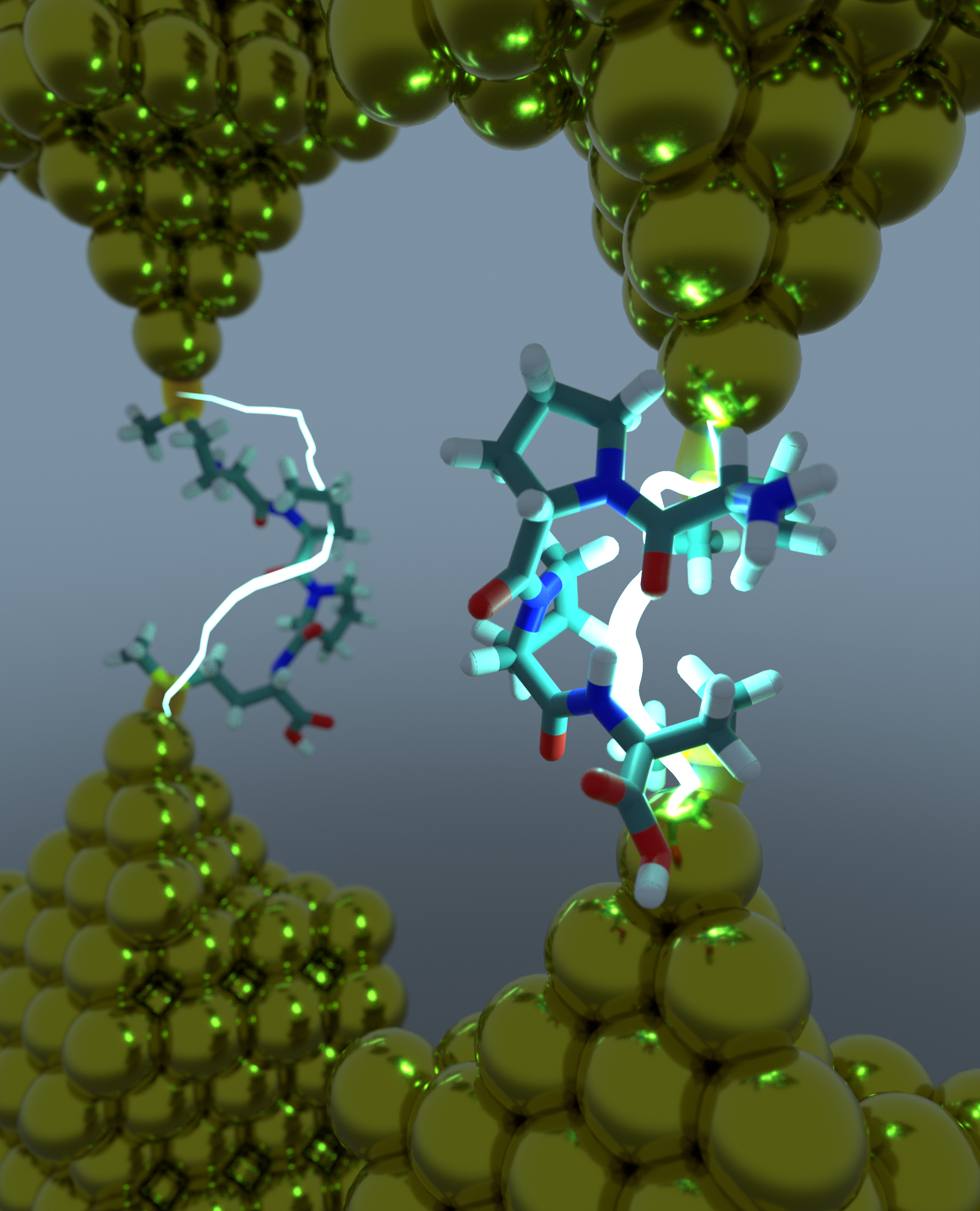
Proteins play a key role in electron transport processes in biology, but the structure-function relationships governing the electronic properties of peptides are not fully understood. We use a combination of single molecule experiments, molecular dynamic (MD) simulations, and quantum mechanics (QM) calculations to show that the electronic properties of peptides critically depend on the conformational flexibility of peptide backbones. A high-conductance state arises due to a defined secondary structure (beta turn or 310 helices), whereas a low-conductance state occurs for extended peptide structures. We further investigated the role of solvent, chirality, hydrogen bonding interactions, and steric constraints on peptide electron transport. First, our results show that water is an effective solvent for facilitating electron transport pathways through hierarchical secondary structures relative to other polar or non-polar solvents. Second, peptide sequences containing an -NH (amine) group exhibit higher conductance values due to increased rigidity, the presence of hydrogen bonding, and steric constraints compared to -NR groups, where R is an aromatic or alkyl group. These results indicate that peptides are more conductive than to peptoids of similar molecular length. Third, we introduced stereochemical alterations in alanine- and tyrosine-based peptides. Our results show that heterochiral tyrosine peptides—where non-terminal amino acids have differing chirality— result in enhanced electron transport along the molecular length due to enhanced pi-stacking interactions between the aromatic side chains of tyrosine residues. In contrast, heterochiral alanine peptides show enhanced electron transport via the secondary structure pathway due to favorable β-turn conformations. Overall, our results show that peptide chemistry, solvent, hydrogen bonding, steric constrains, and chirality play important distinct and roles peptide electron transport. These results can be utilized as design guidelines for understanding electron transport in more complex peptide or protein sequences, which could play a role in the design of next generation bioelectronic devices.