2025 AIChE Annual Meeting
(51f) Understanding the Active Center of Na-Modified Iron-Based Catalysts for Light Olefin Production during CO2 Hydrogenation
Authors
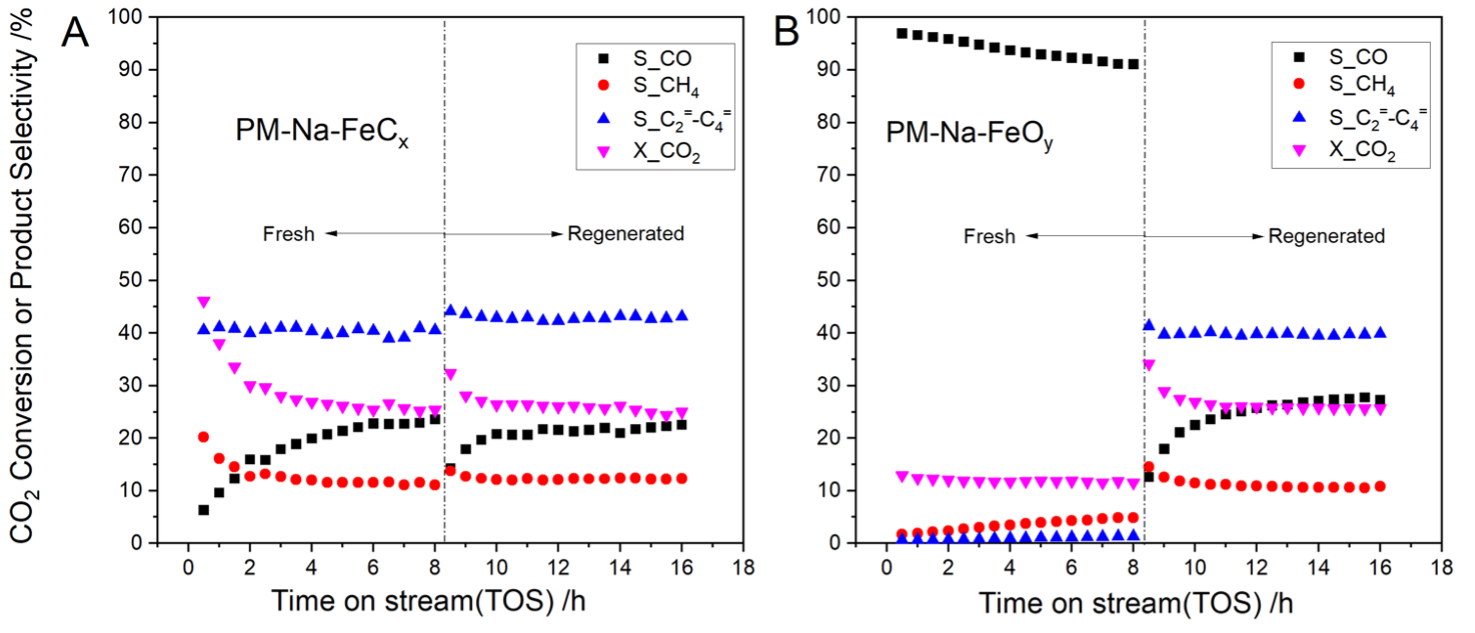
Nanosized Fe5C2 was produced by carburizing nano-Fe2O3 (NANOCAT, 255 m²/g) using syngas, and nanosized Fe3O4. The Na-modified catalysts (PM-Na-FeCx or PM-Na-FeOy) were prepared by grinding Na2CO3 with Fe3O4 or Fe5C2 (nFe:nNa = 1:0.02 molar). CO2 hydrogenation was conducted in a continuous reactor with 1g of catalyst at 20 bar, 325°C. Catalysts were extensively characterized before and after reaction and regeneration (re-carburization).
The nanosized Fe5C2 nano-catalyst shows high FTS activity, while Fe3O4 only exhibits RWGS reactivity. Fe5C2 is easily oxidized to Fe3O4 during CO2 hydrogenation, leading to decreased CO2 conversion and increased CO selectivity. Re-carburization restores its activity and selectivity. A physical mixture of Fe5C2 and Na2CO3 enhances light olefin selectivity, while Fe3O4 and Na2CO3 alone are ineffective. Carburizing Fe3O4 to Fe5C2 and combining it with Na2CO3 improves olefin yield during CO2 hydrogenation (see Figure 1), confirming that the active site is the interface between iron carbide and Na. However, Na2CO3 promotes phase oxidation and inhibits hydrogen dissociation, reducing methanation and FTS activity, so Na2CO3-modified Fe5C2 shows a higher selectivity for CO in light olefin production.