2025 AIChE Annual Meeting
(611c) Uncovering Mechanisms of Interleukin-4 Biology Using a Novel Cytokine Mimetic
Authors
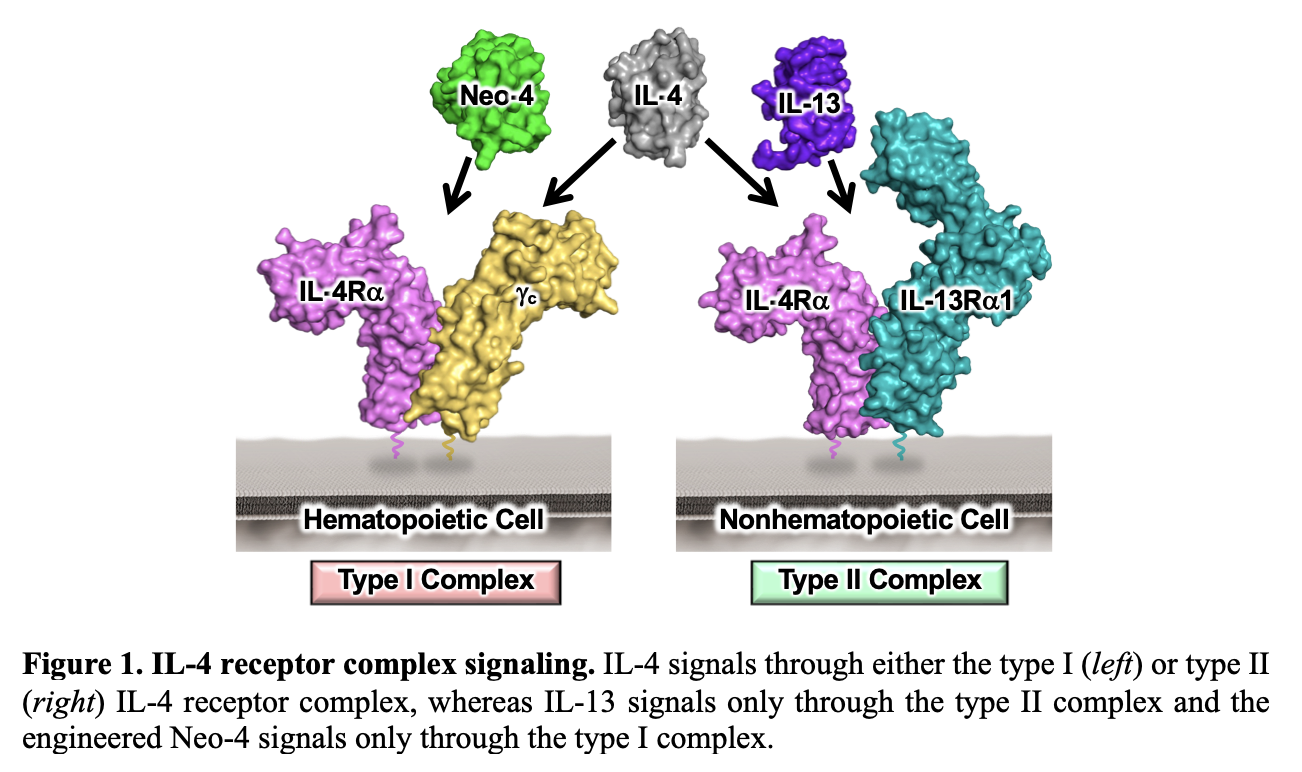
IL-4 signals through two distinct heterodimeric receptor complexes, denoted type I and type II (Figure 1). Within these complexes IL-4 engages its specific receptor chain, IL-4 receptor alpha (IL-4Rα), in combination with either the common gamma (γc) chain (type I complex) or IL-13Rα1 (type II complex).3 A related cytokine, IL-13, signals exclusively through the type II receptor complex, and exhibits similar modulatory effects yet distinct cellular functions compared to IL-4.4,5 Harnessing the beneficial activities and minimizing the pathogenic responses of IL-4 for therapeutic design requires elucidation of signaling, gene expression, cell functions, and in vivo effects of type I versus type II complex activity. However, to date, it has been impossible to untangle the unique biology since no natural cytokine signals exclusively through the type I complex. To overcome this challenge, our team developed a de novo computationally designed IL-4 mimetic (denoted Neo-4) that exclusively signals through the type I complex.6 Using this unique reagent, the goal of this project is to identify how type I and type II IL-4 receptor complexes mediate differential biological responses following stimulation by IL-4, IL-13, and Neo-4. The novel insights gained from this research will in turn inform the development of targeted cytokine-based immunotherapies.
To express IL-4, IL-13, and Neo-4, genes encoding these constructs were cloned into a mammalian expression vector, which were then transiently transfected into Expi293 human embryonic kidney (HEK) cells. The secreted proteins were harvested from cell supernatants by Protein G affinity chromatography followed by size-exclusion chromatography using a fast purification liquid chromatography (FPLC) instrument. Flow cytometry-based signaling studies showed that Neo-4 strongly activated signal transducer and activator of transcription 6 (STAT6) on hematopoietic cells mainly expressing type I receptors (“type I-biased”), such as B-cells. In contrast, Neo-4 does not induce STAT6 activation on non-hematopoietic cells predominantly expressing type II receptor (“type II-biased”), such as fibroblasts. Cells with both type I and type II receptors (“mixed”), such as macrophages, showed intermediate STAT6 activation following Neo-4 stimulation. To assess the behavior of these cytokines in the context of a functional immune system, IL-4, IL-13, and Neo-4 were fused to mouse serum albumin for half-life extension and administered intraperitoneally (i.p.) into immunocompetent mice infected with lipopolysaccharide (LPS) to model acute respiratory distress syndrome (ARDS). Measurements of lung function (airway resistance, dynamic compliance, and diffusion capacity) revealed that all three cytokines elicit similar functional effects despite their unique receptor complex usage properties. Meanwhile, reduced levels of proinflammatory cytokines and attenuated Th2 inflammatory response (neutrophil proliferation) were observed in the bronchoalveolar lavage fluid (BALF) of mice treated with Neo-4 versus IL-13, indicating that type I but not type II IL-4 receptor complex mitigates pathogenic inflammation in the context of respiratory distress. Overall, use of our computationally engineered IL-4 mimetic deepens our understanding of immune biology and provides a roadmap for designing cytokine-based interventions to ameliorate lung inflammation for a variety of disease applications.
References
(1) Junttila, I. S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. https://doi.org/10.3389/fimmu.2018.00888.
(2) Wynn, T. A. Type 2 Cytokines: Mechanisms and Therapeutic Strategies. Nat Rev Immunol 2015, 15 (5), 271–282. https://doi.org/10.1038/nri3831.
(3) LaPorte, S. L.; Juo, Z. S.; Vaclavikova, J.; Colf, L. A.; Qi, X.; Heller, N. M.; Keegan, A. D.; Garcia, K. C. Molecular and Structural Basis of Cytokine Receptor Pleiotropy in the Interleukin-4/13 System. Cell 2008, 132 (2), 259–272. https://doi.org/10.1016/j.cell.2007.12.030.
(4) Heller, N. M.; Qi, X.; Junttila, I. S.; Shirey, K. A.; Vogel, S. N.; Paul, W. E.; Keegan, A. D. Type I IL-4Rs Selectively Activate IRS-2 to Induce Target Gene Expression in Macrophages. Sci. Signal. 2008, 1 (51). https://doi.org/10.1126/scisignal.1164795.
(5) Junttila, I. S.; Creusot, R. J.; Moraga, I.; Bates, D. L.; Wong, M. T.; Alonso, M. N.; Suhoski, M. M.; Lupardus, P.; Meier-Schellersheim, M.; Engleman, E. G.; Utz, P. J.; Fathman, C. G.; Paul, W. E.; Garcia, K. C. Redirecting Cell-Type Specific Cytokine Responses with Engineered Interleukin-4 Superkines. Nat Chem Biol 2012, 8 (12), 990–998. https://doi.org/10.1038/nchembio.1096.
(6) Yang, H.; Ulge, U. Y.; Quijano-Rubio, A.; Bernstein, Z. J.; Maestas, D. R.; Chun, J.-H.; Wang, W.; Lin, J.-X.; Jude, K. M.; Singh, S.; Orcutt-Jahns, B. T.; Li, P.; Mou, J.; Chung, L.; Kuo, Y.-H.; Ali, Y. H.; Meyer, A. S.; Grayson, W. L.; Heller, N. M.; Garcia, K. C.; Leonard, W. J.; Silva, D.-A.; Elisseeff, J. H.; Baker, D.; Spangler, J. B. Design of Cell-Type-Specific Hyperstable IL-4 Mimetics via Modular de Novo Scaffolds. Nat Chem Biol 2023, 19 (9), 1127–1137. https://doi.org/10.1038/s41589-023-01313-6.