2025 AIChE Annual Meeting
(343e) Ultrasensitive and Long-Lasting Bioluminescence Immunoassay for Point-of-Care Viral Antigen Detection
Authors
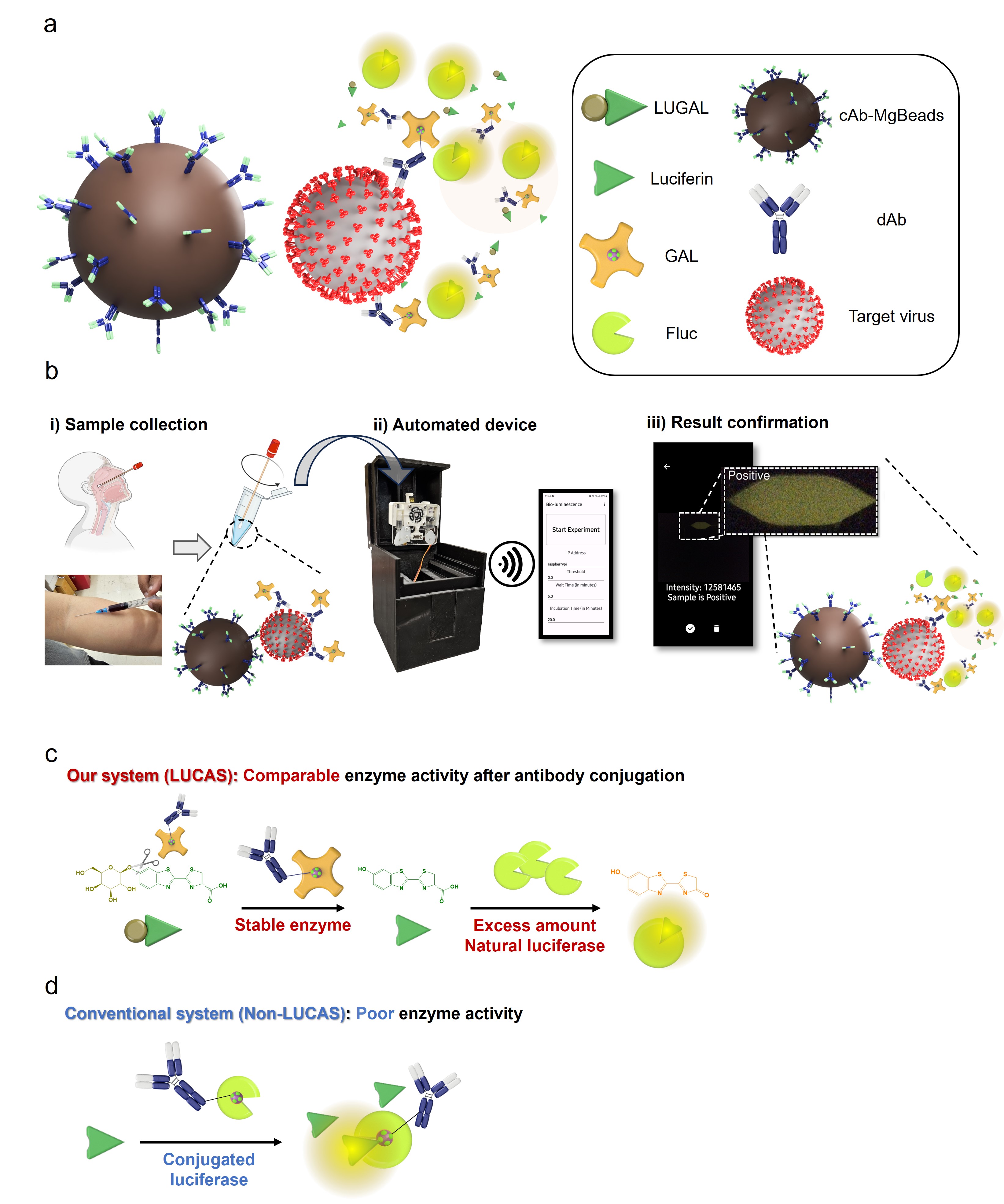
Figure | LUCAS schematic overview in the detection module. a, Target virus capture is achieved using a MgBeads-based immunoassay, where cAb-MgBeads and dAb-GAL capture the viruses. A strong bioluminescence signal is produced via an enzyme cascade reaction mediated by GAL and Fluc. b, Overview of a user-friendly automated diagnostic device and process: i) Collecting a virus sample, mixing it with assay materials, and transferring the mixture to the automated device. ii) Initiating the automated assay by pressing the start button on the smartphone application, with bioluminescence signal detection using a CMOS sensor inside the automated device. iii) Displaying the infection result on the smartphone application. c, Conjugation of GAL to antibodies, enabling GAL to maintain its activity, facilitating the conversion of LUGAL to luciferin, followed by natural Fluc-mediated oxidation, resulting in highly sensitive bioluminescence. d, Comparison with non-LUCAS systems, where Fluc is linked to antibodies, generating bioluminescence in the presence of luciferin. Abbreviations: LUGAL = D-Luciferin-6-O-beta-D-galactopyranoside, cAb-MgBeads = capture antibody conjugated magnetic beads, dAb = detection antibody, GAL = β-galactosidase, Fluc = firefly luciferase.