2025 AIChE Annual Meeting
(719c) Ultra High Concentration Antibody Formulations Enabled Via Thermostable Ionic Liquids
Authors
Concentrated protein therapeutic formulations have garnered significant attention in both commercial and research efforts due to patient-favored, facile administration via subcutaneous injections. However, they face challenges associated with their high viscosity and potential for aggregation induced by intermolecular electrostatic and hydrophobic protein-protein interactions. Meeting the injectability profile (<20 cP) thereby necessitates addition of inactive ingredients to screen protein-protein interactions (PPIs) capable of preventing aggregation via a shielding effect.
Current inactive ingredient chemistries include mixtures of polysorbate surfactants, amino acids, disaccharides, salts, and hyaluronidase enzymes designed to mediate PPIs and improve transport. However, clinical formulations retain a narrow colloidal stability window due to changes in molecular chemistry upon physical and thermal stress, as well as a suboptimal bioavailability (50-85%). We seek to simplify and stabilize these formulations through use of ionic liquids (ILs), a liquid salt (Tm < 100°C) chemistry permitting tunable charge and amphiphilicity profiles via cation-to-anion ratio control.
Materials and Methods:
Herein, we synthesized biocompatible cholinium and organic acid based ILs in either 2:1, 1:1, or 1:2 cation-to-anion ratios and assessed their viscosity profiles initially with IgG antibody at a high concentration (90 mg/mL) followed by an ultra-high concentration (230 mg/mL). Using assays assessing functional (binding efficiency) and structural (circular dichroism) stability for up to 4 months in room temperature storage, candidates for in vivo experimentation were down selected for studies at both 90 mg/mL and 230 mg/mL, with final candidates tested against a clinically deployed monoclonal antibody (mAb).
Results and Discussion:
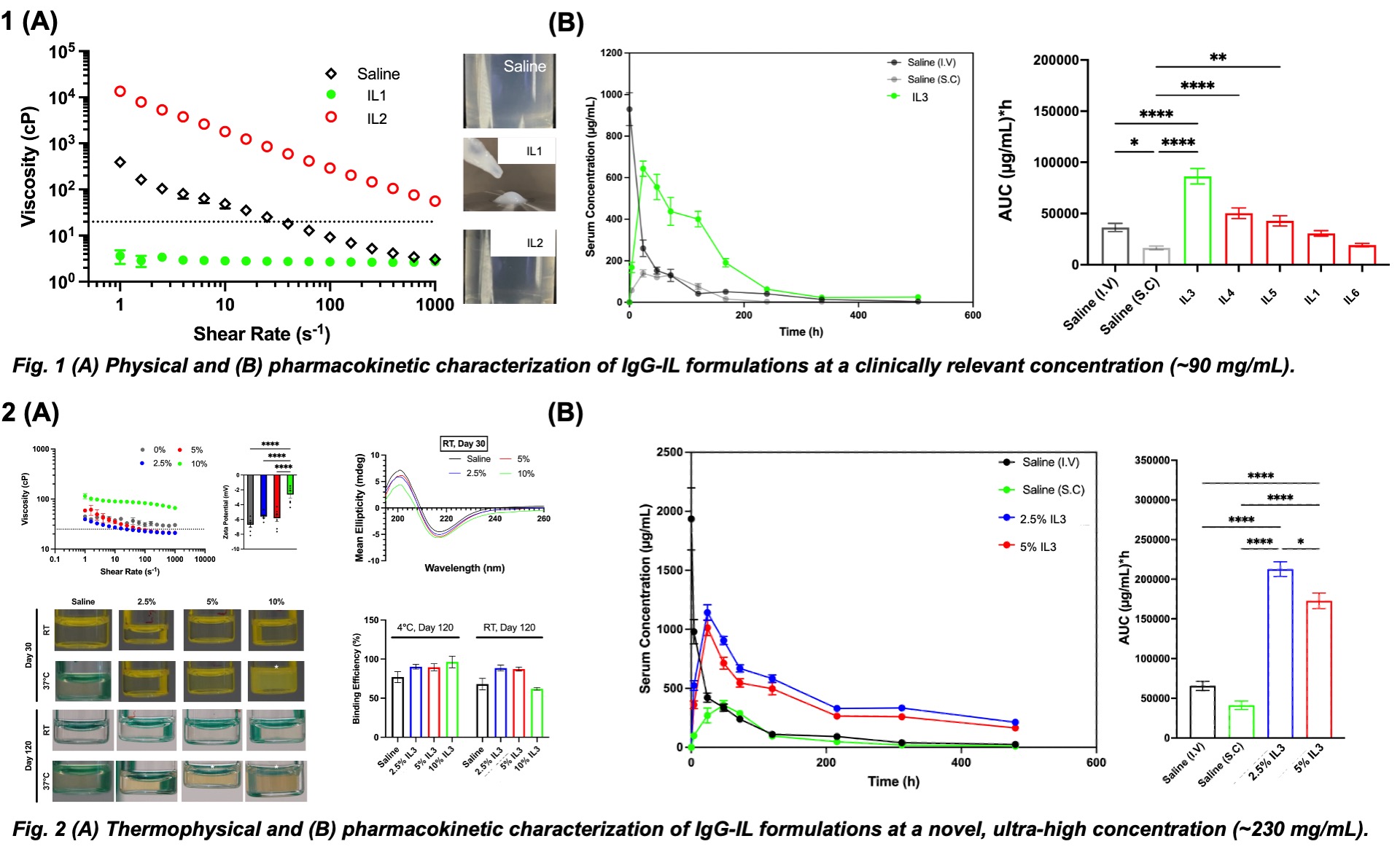
24 unique ionic liquids (ILs) were synthesized with cations and anions presenting a wide range of hydrophobic properties. Viscosities of candidate IgG-IL formulations at 90 mg/mL decreased monotonically with increasing shear rates to values 10-fold lower than saline control, while non-candidates exhibited a 10-fold decrease relative to control (Fig. 1A). Overall, 6 of 24 formulations passed the viscosity threshold of 20 cP and were selected for initial in vivo pharmacokinetic screening. At a clinically relevant concentration (~90 mg/mL), all IgG-IL formulations led to improved bioavailability compared to the subcutaneous saline formulation, with one exhibiting over a 5-fold increase in bioavailability compared to subcutaneous saline injection (Fig. 1B).
With a singular candidate following robust in vitro and in vivo screening, we scaled up concentration to be ultra-high (~230 mg/mL), as we aimed to further reduce subcutaneous dosing events required to maintain equivalence with an intravenous therapy. However, this presented a material challenge as we observed viscosity values exceeding a 20cP threshold value (Fig. 2A). We deduced that this was due to a protein “salting in” and “salting out” phenomena, where there was a non-linear relationship between IL salt concentration and formulation viscosity (i.e. solubility) due to a competition between free water and IL ions to bind to protein surface. In conjunction with high viscosity values at IL concentrations (5-10% v/v) nearing a critical threshold, we observed reduced thermal stability in both room temperature and 37°C storage conditions. Interestingly, we noticed that lower IL concentrations (2.5% v/v) presented a combination of lower viscosity values (<20cP) as well as near 100% functional binding efficiency following room temperature storage for 4 months (Fig. 2B). Coupled with a 4-fold increase in serum accumulation versus both intravenous and subcutaneous saline controls (Fig. 2C), this presents a landmark opportunity for end-user clinical translation.
Conclusion:
In this study we deployed a novel ionic liquid formulation with both a clinically relevant (~90 mg/mL) and a novel, ultra-high (~230 mg/mL) concentration of antibody for subcutaneous delivery. We not only demonstrated its ability to withstand high degrees of thermal stress at both doses, but also an ability to far improve accumulation in circulation versus control intravenous and subcutaneous formulations. This was accomplished through a fundamental lens of protein salting-in and salting-out, where sufficient IL is presented in solution to solubilize antibody without induction of protein-protein interactions driving aggregation through a shorter electrostatic screening length. Coupled with the facile and simplistic synthesis through use of biocompatible ions, this IL holds massive clinical potential in the field of scalable, safe, and effective therapeutic delivery.