2025 AIChE Annual Meeting
(406e) Tuning the Electronic Structure of Mxene As a Support for Electrochemical CO2 Reduction Catalysts
Author
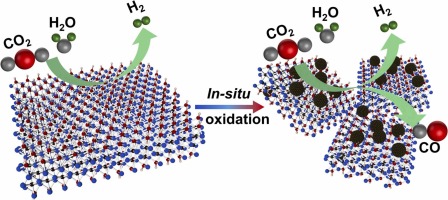
Herein, we report that the electronic structure of Ti3C2Tx can be tuned by introducing cations, with particular emphasis on the in-situ formation of Ag nanoparticles (NPs) via Ag+ loading (with Ti3C2Tx as a de facto reducing agent). Controlling the electronic structure of Ti3C2Tx is critical for balancing the competition between CO2RR and HER, thereby tuning overall selectivity and activity. Our systematic investigations reveal that cations with lower reduction potentials (eg. Na+, Co2+, Ni2+) do not significantly alter CO2RR performance, whereas cations with higher reduction potentials (eg. Cu2+, Ag+) can modify Ti3C2Tx’s electronic structure. Specifically, in-situ reduced Ag NPs on Ti3C2Tx yield a higher Ti oxidation state and increased Ti–O bond content compared to samples containing physically mixed Ag NPs loaded onto MXene. This electronic restructuring suppresses competing HER and enhances the CO Faradaic efficiency (FE). For example, the CO FE rises from 8.1 % (partial current density -2.0 mA cm-2) to 49.6 % (partial current density -20.1 mA cm-2) at -1.4V vs. RHE. Additionally, the ratio of CO to H2 in the syngas product can be controllably adjusted from 1:3 to 1:1.
This work contributes to the fundamental understanding of MXene-based catalysts and provides a strategic pathway to systematically tune the CO2RR/HER balance for efficient carbon utilization. Finally, this presentation will give insights into the outlook for MXene as a tunable electrocatalyst support.