2025 AIChE Annual Meeting
(309f) Tuning Electrochemical Hydrodeoxygenation Pathways of Bio-Oil Compounds with Bimetallic Pt Catalysts
Authors
Electrochemical upgrading represents a promising route for converting bio-oils into valuable fuels or chemicals under mild operating conditions. Among these, electrochemical hydrodeoxygenation (EC-HDO) has gained attention as an effective approach to stabilize bio-oils.[2] This process uses hydrogen generated at the anode to reduce oxygen content and enhance the hydrogen content of bio-oils. EC-HDO offers several advantages, including low-temperature operation (<80 °C), ambient pressure, and the in-situ generation of hydrogen—eliminating the need for external hydrogen supply.[2] Additionally, EC-HDO presents opportunities for direct integration with intermittent renewable energy sources, such as wind and solar.
This study focuses on improving the reaction rate and product selectivity in the conversion of phenol to cyclohexane by establishing structure-reactivity relationships in mono- and bimetallic catalysts. Phenol, a representative bio-oil model compound, was electrochemically upgraded in a custom-built H-cell, with the anode and cathode separated by a Nafion® 117 membrane. Electrochemical testing was performed under both potentiostatic and galvanostatic conditions. A range of mono- and bimetallic catalysts—including Pt, Ru, Ni, Co, Cu, PtNi, PtCu, PtCo, and PtRu—were synthesized using incipient wetness impregnation on Vulcan XC-72R carbon support. These catalysts were characterized via nitrogen adsorption-desorption, transmission electron microscopy (TEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and inductively coupled plasma atomic emission spectroscopy (ICP-AES).
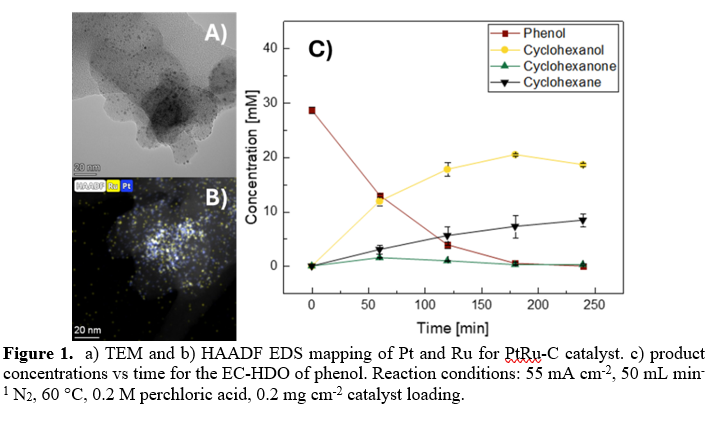
Catalyst characterization confirmed successful preparation of PtRu, Pt, and Ru catalysts. TEM and HAADF-EDS mapping (Figure 1a, 1b) of the PtRu sample confirmed uniform deposition of Pt and Ru on the carbon support. Galvanostatic EC-HDO experiments were conducted to evaluate the influence of active metal species on the conversion and selectivity of phenol. Initial results using the PtRu-C catalyst showed a cyclohexane selectivity of 31% after 4 hours of electrolysis (Figure 1c). Follow-up tests using individual Pt and Ru catalysts were performed to assess the contribution of each metal. The PtRu catalyst exhibited superior performance, achieving the highest selectivity (PtRu: 31%, Pt: 24%, Ru: 10.5%), complete phenol conversion (PtRu: 100%, Pt: 87%, Ru: 41.1%), and the highest overall efficiency (PtRu: 35%, Pt: 32%, Ru: 11%).
References
- J.R. Page, Z. Manfredi, S. Bliznakov, J.A. Valla, Recent Progress in Electrochemical Upgrading of Bio-Oil Model Compounds and Bio-Oils to Renewable Fuels and Platform Chemicals, Materials 16 (2023) 1–33.
- J.R. Page, A. Pophali, T. Kim, J.A. Lopez-Ruiz, S. Bliznakov, J.A. Valla, Effect of Pt and Ru-based catalysts on the electrochemical hydrodeoxygenation of phenol to cyclohexane, Catal Sci Technol 14 (2024) 5559–5573. https://doi.org/10.1039/D4CY00634H.