2025 AIChE Annual Meeting
(401m) Towards a Versatile Direct Air Capture Process: Carbon Recovery Performance of Supported Ionic Liquid Membranes under various Humidity Conditions
Authors
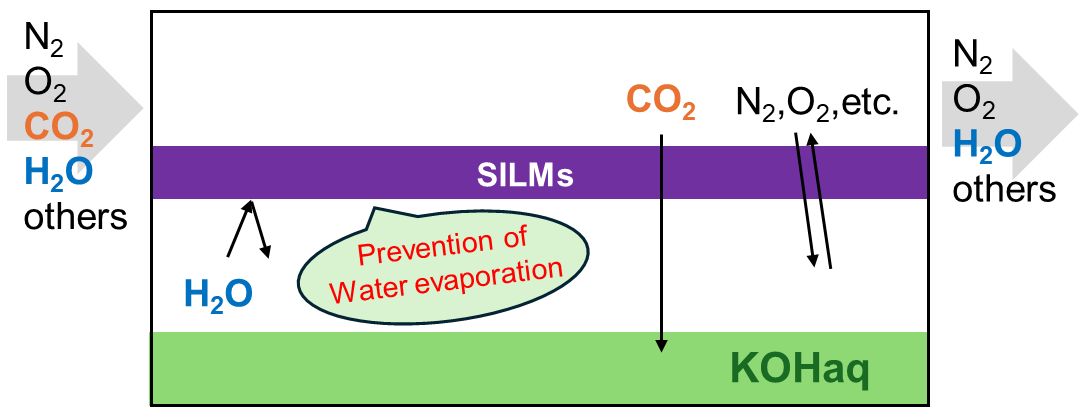
In this study, we conducted CO2 absorption experiments from atmosphere under various humidity conditions using supported ionic liquid membranes (SILMs) and aqueous potassium hydroxide (KOH) as the absorbent. KOH solution was selected due to its non-volatility, high CO2 affinity, and resistance to chemical degradation under ambient conditions. The membrane used was a hydrophobic SILM supported on a porous polymer substrate. To examine the membrane's tolerance to atmospheric humidity, we compared its performance against a hydrophilic SILM and a blank (support-only) membrane. Importantly, all membranes used the same support material PTFE to isolate the effects of the liquid phase and surface characteristics. To minimize the impact of external fluctuations, we measured the ambient CO2 concentration for each run and corrected the observed absorption performance based on those values [3].
Consequently, the raw CO2 absorption performance exhibited no noticeable trends with respect to type of ionic liquids, making it difficult to interpret the effects of humidity and ionic liquid type. To eliminate the influence of ambient CO2 concentration fluctuations, the absorption rates were normalized by the inlet CO2 concentration measured during each experiment. After applying this correction, the differences in CO2 absorption performance among membranes with different humidity levels and ionic liquids largely disappeared. These results indicate that, under the conditions tested, membrane hydrophobicity and atmospheric humidity were not dominant factors affecting CO2 absorption performance, and that proper correction for environmental variables is essential for fair membrane evaluation. We attribute the observed uniformity in CO2 absorption performance to the use of aqueous KOH, which functioned as a chemically stable, non-volatile absorbent that enabled clear comparison across membrane types and humidity conditions. Its insensitivity to water vapor dilution made it particularly well-suited for isolating membrane-specific effects.
The fact that CO2 absorption performance remained consistent across different humidity levels and membrane types suggests that these SILMs can offer reliable operation even in challenging ambient conditions by suppressing solvent evaporation while maintaining CO2 capture performance. Also, these findings highlight the importance of carefully selecting test conditions when evaluating membrane performance and suggest that, under corrected and controlled environments, the role of membrane hydrophobicity may be less significant than previously assumed. Ultimately, this work contributes to the development of DAC systems that are robust to environmental variability, expanding their potential applicability to a broader range of climates.