2025 AIChE Annual Meeting
(504f) Towards Sustainable Downstream Processing with Continuous Crystallization
Authors
While most large-scale protein crystallization processes have been implemented in batch, one effective strategy to address poor reproducibility and scalability often observed is to shift to continuous mode. Mixed-suspension mixed-product removal crystallizer (MSMPRC), based on continuous stirred tanks reactors (STRs), and tubular reactors are the main types of continuous crystallizers [4]. Though tubular reactors overcome most STRs limitations (e.g. low micromixing efficiency, high shear rate near the impellers), they are not suitable for the slow kinetics of protein crystallization (e.g., excessive tube length, clogging).
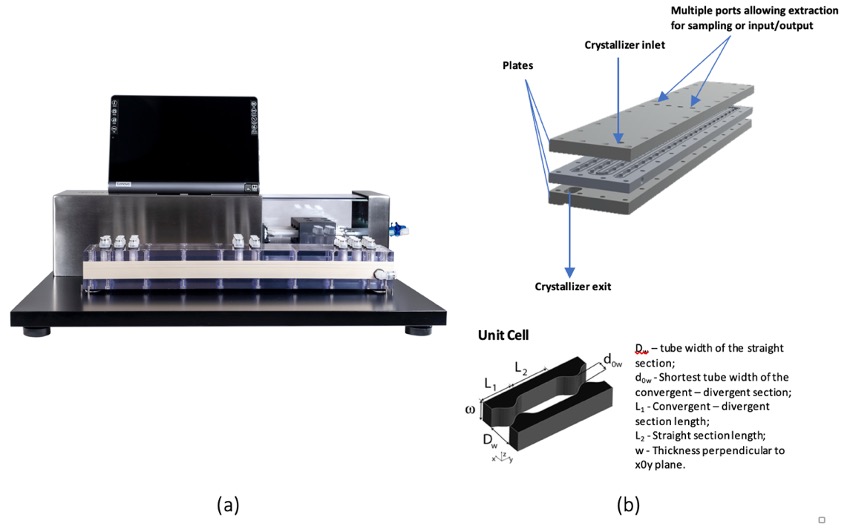
In the present work, an innovative platform for continuous protein crystallization is presented. It consists in a cutting-edge modular oscillatory flow plate reactor (MOPR) [5] along with process analytical technology (PAT) tools (e.g. UV-Vis spectroscopy and imaging) (Figure 1). While MOPR offers enhanced hydrodynamic and transport properties, accurate data collection through PAT tools allows identifying critical steps for further process control. A model protein is studied (i.e., insulin), where the influence of process parameters (e.g., initial supersaturation, residence time, oscillation conditions) is assessed to meet specific requirements in terms of crystallization kinetics and crystal attributes (crystal size distribution-CSD, shape).
Figure 1. Oscillatory flow plate unit (a) comprising a full controlled oscillatory unit and a Planar Oscillatory Flow Crystallizer (Planar-OFC) and (b) overview of the three dimensional Planar-OFC geometry and its parametric variables.
The results obtained clearly demonstrate the possibility of using this type of reactor in the continuous crystallization of proteins, with the ultimate goal of boosting its implementation at the industrial scale for the downstream processing (DSP) of proteins, having a tremendous impact on the manufacturing costs and environmental impact of DSP.
References
[1] R. dos Santos, A. L. Carvalho, and A. C. A. Roque, Biotechnol. Adv., 2017, 35, 1, 41–50.
[2] F. Castro, N. R. da Silva, S. C. Silvério, L. F. Ballesteros, and J. A. Teixeira, Current Developments in Biotechnology and Bioengineering, R. Sirohi, A. Pandey, M. J. Taherzadeh, and C. Larroche, Eds. Elsevier, 2022, 455–495.
[3] C. N. Nanev, Prog. Cryst. Growth Charact. Mater., 2020, 66, 2.
[4] S. Pu and K. Hadinoto, Chem. Eng. Res. Des., 2020, 160, 89–104.
[5] A. Ferreira, F. Rocha, J. Teixeira, and F. Castro, EP 3439773 B1, 2022.
[6] F. Castro, A. Ferreira, J. A. Teixeira, and F. Rocha, Cryst. Growth Des., 2016, 16, 7. 3748–3755.
Acknowledgements
This work was supported by national funds through FCT/MCTES (PIDDAC): UIDB/04469/2020 (CEB) (DOI: 10.54499/UIDB/04469/2020) and LA/P/0029/2020 (LABBELS), 2023.11352.PEX (DOI: 10.54499/2023.11352.PEX); LEPABE, UIDB/00511/2020 (DOI: 10.54499/UIDB/00511/2020) and UIDP/00511/2020 (DOI: 10.54499/UIDP/00511/2020) and ALiCE, LA/P/0045/2020 (DOI: 10.54499/LA/P/0045/2020).