2025 AIChE Annual Meeting
(375e) Towards the Selective Dissolution of Lithium from the Cathodes of NMC-Type Lithium-Ion Batteries By Leaching Under Oxidizing Conditions
The effect of potential is best understood by considering the delithiation mechanism in LiBs: while the battery charges, the transition metals in the cathode are oxidized, accompanied by an evolution of the crystalline structure. Driven by a charge transfer, lithium is expelled in the electrolyte, resulting in a transition of the initial rhombohedral O3 metal-oxide crystal to a O3' phase, and then an intermediate H13 crystalline phase composed of both lithium-rich and lithium-free lattices. The final stage of O1 delithiation corresponds to the full charge of a working LiBs.
We based our work on the hypothesis that lithium from a BM powder could be selectively recovered in an aqueous phase by an oxidation mechanism similar to that which occurs during battery operation. The aim of this paper is to understand and master the operating conditions that control the delithiation process, and apply them to the selective leaching of lithium from BM powders under oxidizing conditions in an aqueous media. The methodology is based on experimental trials using industrial materials, detailed characterization of the leachate and solid residues, and interpretation of the results using thermodynamic equilibrium calculations.
The BM powder was prepared by physical pre-treatment steps (mainly grinding and sieving, without electrode separation or heat treatment). Three 8 kg batches of industrial BM, from different types of LiBs available on the market, were considered in this work. They all have different Ni/Co/Mn ratios, and each contain about 25 wt.% graphite. BM leaching tests were carried out at atmospheric pressure in a 6 L double-shell reactor controlled by a thermostatic bath. The following operating conditions were chosen: T = 40°C, pH set at 6 or 7 by addition of H2SO4, Eh measured and/or controlled between 450 mV and 1140 mV (Eh vs. ESH), for leaching runs of 2 to 48 h. Different oxidants were used: H2O2, KMnO4, and NaClO. Kinetic monitoring of leaching progress was ensured by continuous measurement of the volume of acid consumed and elemental analysis of suspension samples taken during the reaction.
A testing plan was designed to characterize the samples extensively before and after leaching. Metal contents in industrial BM and in leachates filtered on 0.2 µm syringe filters were quantified by inductively coupled plasma optical emission spectroscopy (ICP-OES, HORIBA - JOBIN YVON ULTIMA Expert). The evolution of the crystalline phases in the solids was characterized by X-ray diffraction (XRD, Bruker D8 Advance A25). The morphological properties of the particles were observed by electron scanning microscope (SEM-FEG, JEOL JSM 7100F + EDX Oxford ASDD X-Max 50mm2).
The choice of a pH range between 6 and 7 was based on preliminary thermodynamic equilibrium simulations: to ensure Li selectivity, Li needs to be soluble in the aqueous phase, while other metals should be stable as solid phases. However, the simulations are limited to the simplified case of the LiCoO2 - H2SO4 system, as no model describing Li(Co,Ni,Mn)O2 solid solution has been published yet. Simulation calculations indicated that lithium is the only soluble metal at pH = 7, while the other elements are present as stable solid phases such as Ni(OH)2 or Co3O4 (see Figure 1).
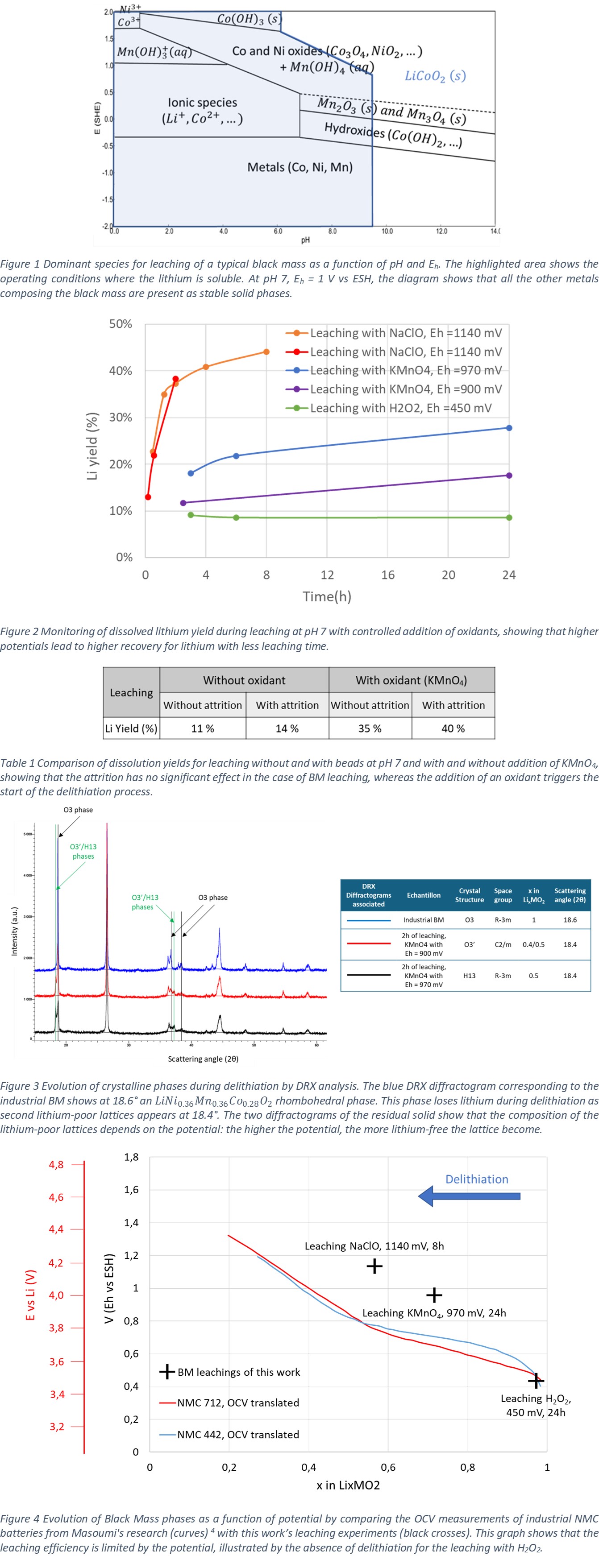
Figure 2 shows the evolution of the delithiation efficiency at different potentials of an BM with average initial composition . Whatever the conditions, about 8-10% of the lithium dissolves very quickly. This is attributed to the proportion of lithium that is not in the cathode crystal lattice when the battery is discharged, but rather is the electrolyte or anode material. For leaching at Eh = 450 mV vs. ESH imposed by addition of H2O2, the Li content in the leachate does not change over time, demonstrating the absence of delithiation under these conditions. For leaching at 900 mV and 970 mV vs ESH by addition of KMnO4, yields of 18% and 28% are obtained after 24 h, indicating partial delithiation of the Nickel-Manganese-Cobalt (NMC) material. Finally, for leaching by addition of NaClO at Eh = 1140 mV vs. ESH, a yield of 44% was obtained after 8 h of leaching. Furthermore, no significant Ni, Mn or Co concentration were measured in the electrolyte in any of the tests, confirming that the conditions used led to selective dissolution of Li. Our tests to date show that delithiation is driven by redox potential increase, and that lithium dissolution under oxidizing conditions is selective towards transition metals at pH around 7.
The evolution of the phases constituting the industrial BM during delithiation was characterized by DRX analysis of the solid leach residues. The diffractograms shown in Figure 3 effectively demonstrate the transition from the initial 03 phase to the intermediate H13 phase, with the gradual disappearance of the major lithium metal oxide phase. A new lithium-depleted phase with a lower lattice parameter and greater atom-to-atom repulsion also appears, characteristic of an increase in the degree of oxidation of the transition metals.
The main point to take away from this paper is that the selective dissolution of lithium can be achieved in water under oxidizing conditions, the limiting factor being the potential imposed by the oxidant. The use of NaClO as a strong oxidant yielded a Li dissolution rate of 44% from industrial black mass powder after 8 h for Eh = 1140 mV vs. ESH. The evolution of the crystalline phases during oxidative leaching was consistent with what is known about crystalline structure evolution during battery usage. Yields of ~50-60% were achieved for longer times and higher NaClO concentrations.
Work in progress concentrates on the search for operating conditions that optimize the efficiency of the delithiation process. The current focus is on the use of sufficiently high potentials to achieve the most favourable potential/pH ranges. Indeed, Figure 4 shows lithium dissolution yield during delithiation as a function of open circuit potential (OCV) from Masoumi's research in 20204. Converting these measurements to V vs. ESH shows that a very strong oxidizing medium is required to achieve full delithiation.
References
(1) Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10 (8), 1107.
(2) Kong, Y.; Takaya, Y.; Córdova-Udaeta, M.; Tokoro, C. Simple and Efficient Selective Extraction of Lithium from Spent Ternary Lithium-Ion Batteries via Oxidation/de-Lithiation Using NaClO. Separation and Purification Technology 2023, 322, 124280.
(3) Dakkoune, A.; Bourgeois, F.; Po, A.; Joulian, C.; Hubau, A.; Touzé, S.; Julcour, C.; Guezennec, A.-G.; Cassayre, L. Hydrometallurgical Processing of Chalcopyrite by Attrition-Aided Leaching. ACS Eng. Au 2023, 3 (3), 195–209.
(4) Masoumi, M. Thermochemical and electrochemical investigations of Li(Ni,Mn,Co)O2 (NMC) as positive electrode material for lithium-ion batteries.