2025 AIChE Annual Meeting
(344a) Time-Dependent Enrichment of Stress-Induced Biomarkers in Plants Using Nanoparticle Protein Coronas
Authors
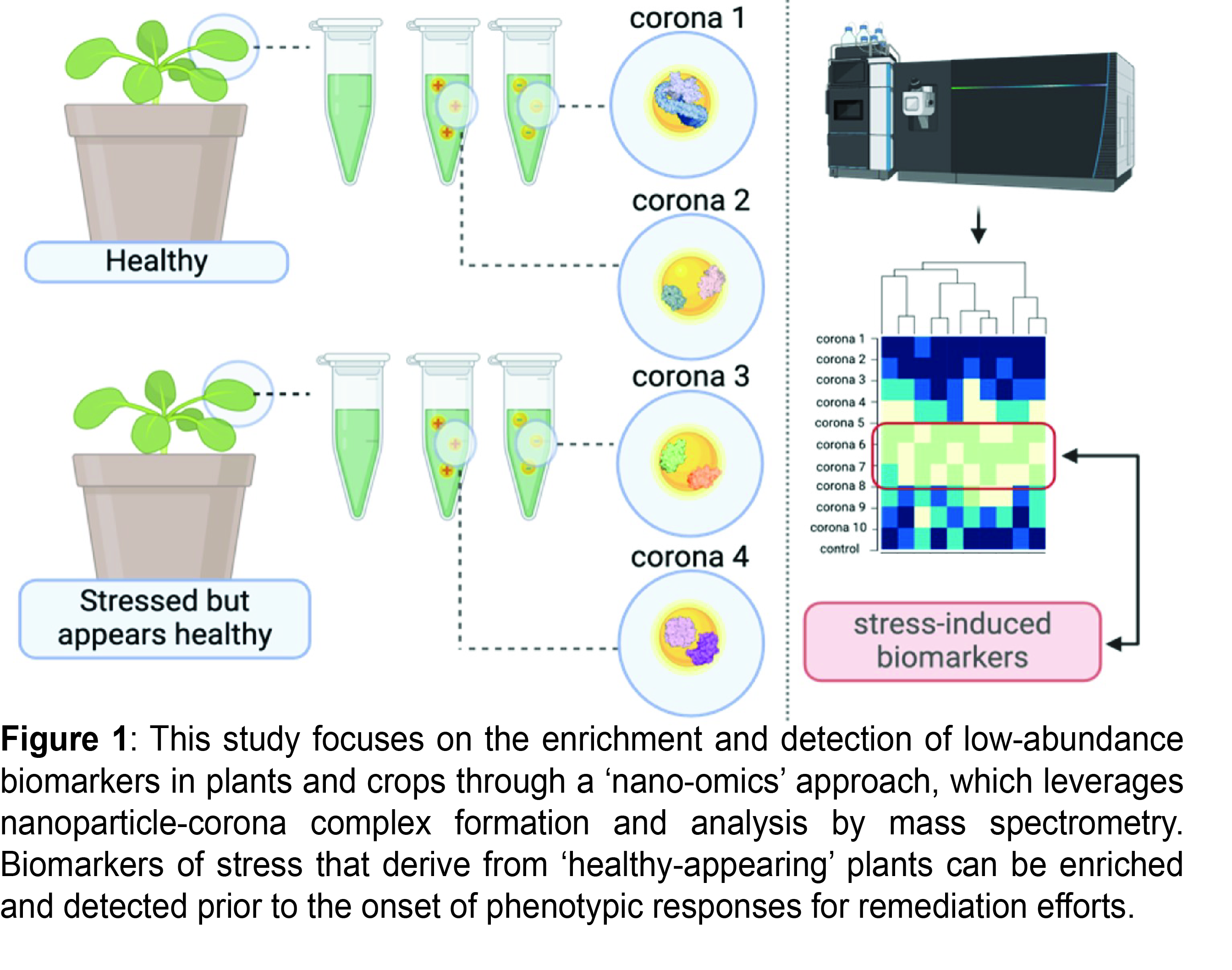
Herein, we exposed plant and crop species to a pathogenic bacterium, Pseudomonas syringae, to prompt a temporal production of multiple stress-induced biomarkers. We collected time-dependent tissue from infected (0.5-, 1-, 3-, and 7-days post infection) Arabidopsis thaliana and Zea mays plants and used 10 nm gold nanoparticles (AuNPs) with different surface charges to enrich and detect stress induced biomarkers through a nano-omics approach (Figure 1). Specifically, our nano-omics approach leverages nanoparticle-corona formation and subsequent analysis with ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS). For plants analyzed with conventional UHPLC-MS/MS alone, molecular stress markers alerted the onset of infection at the same time as visual inspection of the temporal development of phenotypic symptoms ≥3 days post infection. Contrastingly, our highly sensitive nano-omics results show that several unique stress-induced biomarkers, enriched on AuNPs with certain surface chemistries, were detectable in ‘healthy-appearing’ plants at early stages of infection (≤1 day post infection) prior to the onset of phenotypic responses (i.e.: wilting, chlorosis, necrosis). Most interestingly, the sensitivity of our nano-omic approach extended to neighboring ‘distal’ tissues of infected plants. P. syringae, which colonizes the apoplast of directly infected leaves, was itself not detected in ‘distal’ leaves, but our nano-omics approach sensitively detected biomarkers of stress in these neighboring tissues triggered by immune signaling. While this study focused on two different plant species, and one form of biotic stress, we anticipate that this method can also be applied to detect biomarkers in other agriculturally relevant crops induced by a plethora of stressors. Overall, we anticipate that this study will facilitate the development of innovative nanotechnology that leverages nanoparticle-corona complexes for ultra-sensitive and quick identification of diseases and stress in crops, accelerating agricultural productivity and meeting global food demands.