2025 AIChE Annual Meeting
(296f) Thermal Decomposition of Desalination Brine Reject for CO? Capture Applications
MgO + CO2 → MgCO3 , ΔH= -100.9 kJ/mole (1)
This study focuses on magnesium oxide (MgO) formed through the thermal decomposition of brine reject and its potential applications in CO₂ capture and sequestration. The concentrations of the divalent ions magnesium (Mg) in the brine reject solution were determined using inductively coupled plasma optical emission spectroscopy (ICP-OES). Thermal decomposition (calcination) of the brine reject was conducted at 450 °C facilitating the decomposition as per the following reaction:
MgCl2 6H2O → MgO + 2HCl + 5H2O , ΔH =133 kJ/mole (2)
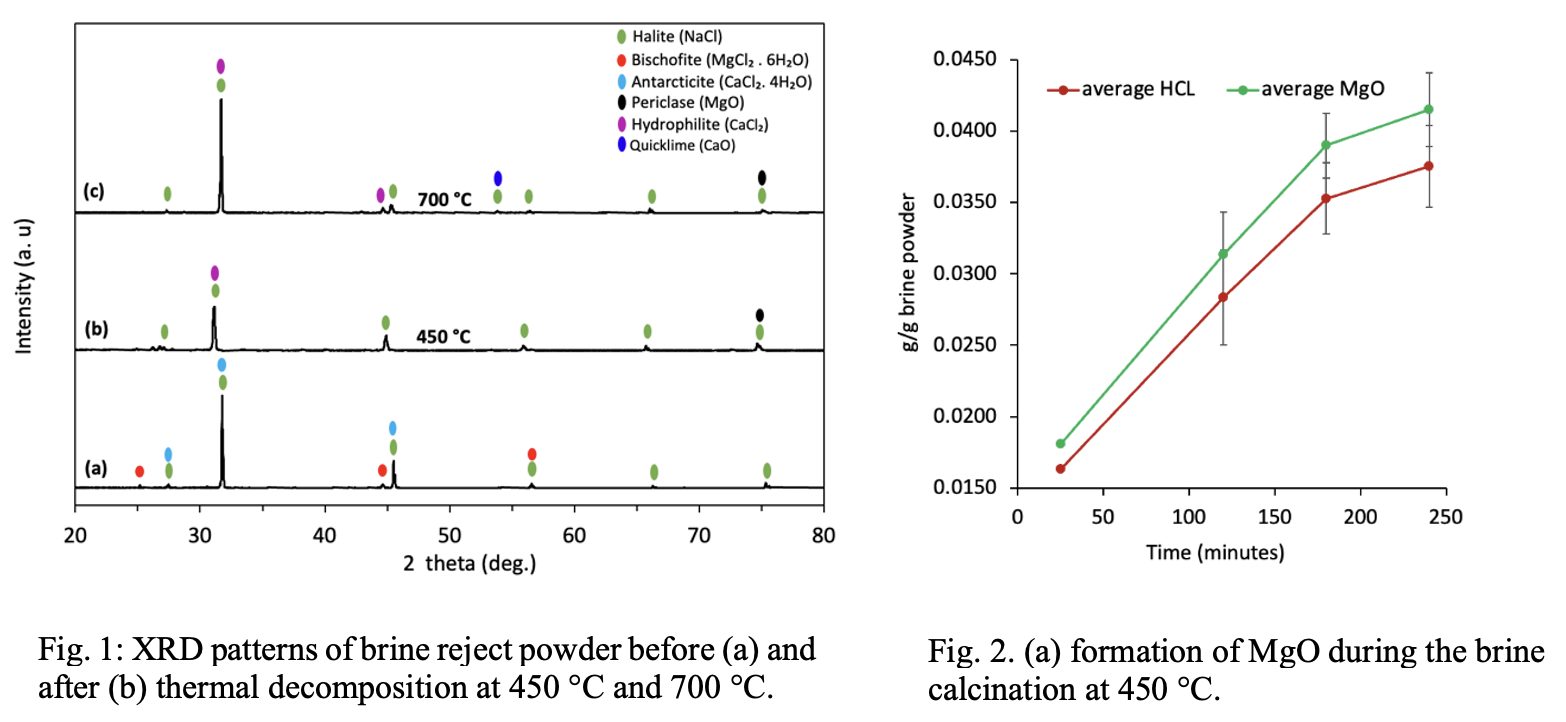
The formation of MgO was confirmed by analyzing the brine phase using X-ray diffraction (XRD). The calcined brine powder porosity, pore size distribution, and surface area were determined using BET analysis. The concentration of magnesium (Mg) in the brine reject was found to be 1439.5 ppm. The X-ray diffraction (XRD) results confirmed the formation of magnesium oxide (MgO) at 450 ℃, evidenced by the complete disappearance of the characteristic peaks of Bischofite (MgCl₂·6H₂O) and the emergence of the MgO peak at 74.67°, as shown in Figures 1a-b. Additionally, the results indicated that the calcined brine had a mesoporous structure, however, as the decomposition temperature increased to 700 °C, a clear sintering occurred, leading to the collapse of the porous structure and transformation into a microporous structure. This was further confirmed by a reduction in surface area from 2.83 m²/g at 450°C to 1.23 m²/g at 700 °C. Following calcination for 4 hours at 450 °C, as shown in Figure 2, the result indicated that the calcined powder contains 4.15 mass % MgO. Accordingly, the calculation based on the stoichiometry of reaction (1) shows that calcined brine reject has the potential to capture and sequester 0.0453 g of CO₂ per gram. If the total global production of brine reject, which is 51,647.5 million m³ per annum, is utilized, this would result in approximately a minimum of 33 Mt of CO₂ captured annually. In regions with high solar radiation year-round, such as the Middle East and North Africa (MENA), concentrated solar thermal energy can be used to drive the highly endothermic decomposition (calcination) of brine reject, as described in reaction (2).