2025 AIChE Annual Meeting
(7b) Theoretical Insight into the Mechanism of Ethylene Production from Methanol in MFI Using Aromatic Co-Catalysts
Authors
Charlton Sullivan - Presenter, Purdue University
Mykela DeLuca, University of Florida
David Hibbitts, University of Florida
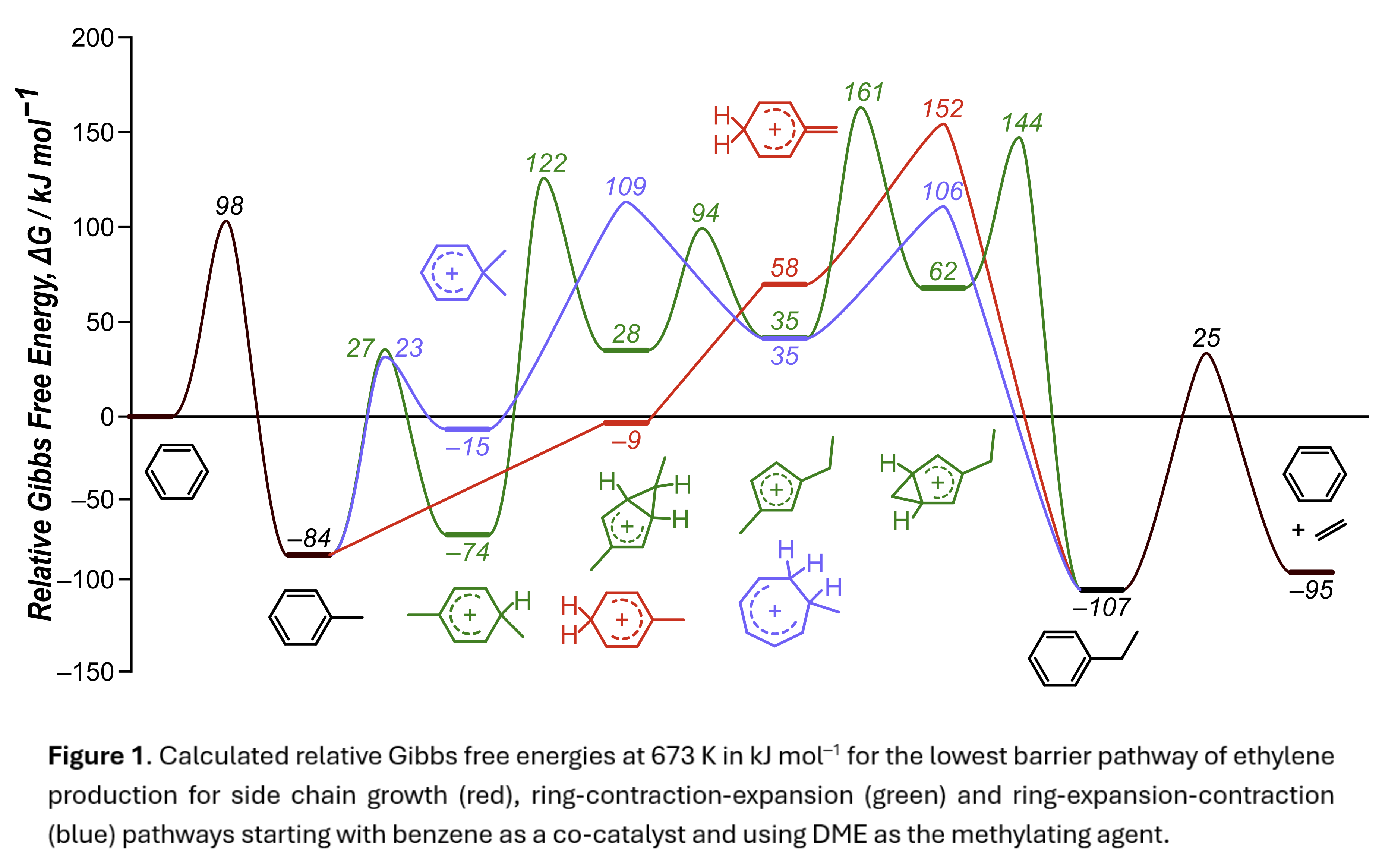
Methanol-to-olefins (MTO) chemistry converts methanol into more valuable light olefins, primarily ethylene and propylene, using Brønsted acid zeolites. Two cycles of MTO chemistry contribute to the production of these light olefins—the olefins cycle, where linear and branched alkanes crack to form olefins, and the aromatic cycle, where aromatics act as co-catalysts and help facilitate the formation of light olefins. We further divide the aromatic cycle into three distinct pathways—the side chain growth mechanism (SC), the paring or ring-contraction-expansion mechanism (RCE), and the ring-expansion-contraction mechanism (REC). In this study, we use density functional theory (DFT) to compare both previously proposed and novel mechanistic pathways to produce ethylene via the aromatic cycle in H-MFI. Results of this analysis with benzene indicates that the highest free energy step (relative to a reference state of benzene + H–Z + 2 DME at 673 K) for each pathway is the methylation of the exocyclic carbon (152 kJ mol‒1) for the SC pathway, the ring expansion (161 kJ mol‒1) for the RCE pathway, and the ring expansion (109 kJ mol−1) for the REC pathway. DFT calculated barriers for pathways involving ethylene elimination from a 5MR in the RCE mechanism are significantly higher than for expansion to a 6MR prior to ethylene elimination, suggesting that ethylene elimination from a 5MR species rarely occurs. These calculations and analyses for benzene can be repeated for larger polymethylbenzene co-catalysts. This data can then be used in conjunction with diffusive barriers of chemical species in MFI to inform kinetic Monte Carlo simulations to determine the most-abundant adsorbed intermediate species within the MFI crystal and to predict observed reaction rates.