2025 AIChE Annual Meeting
(347b) Techno-Economic Analysis of Catalytic Methane Pyrolysis in a Fluidized Bed Reactor with Reactor-Scale Catalyst Deactivation Modeling
Authors
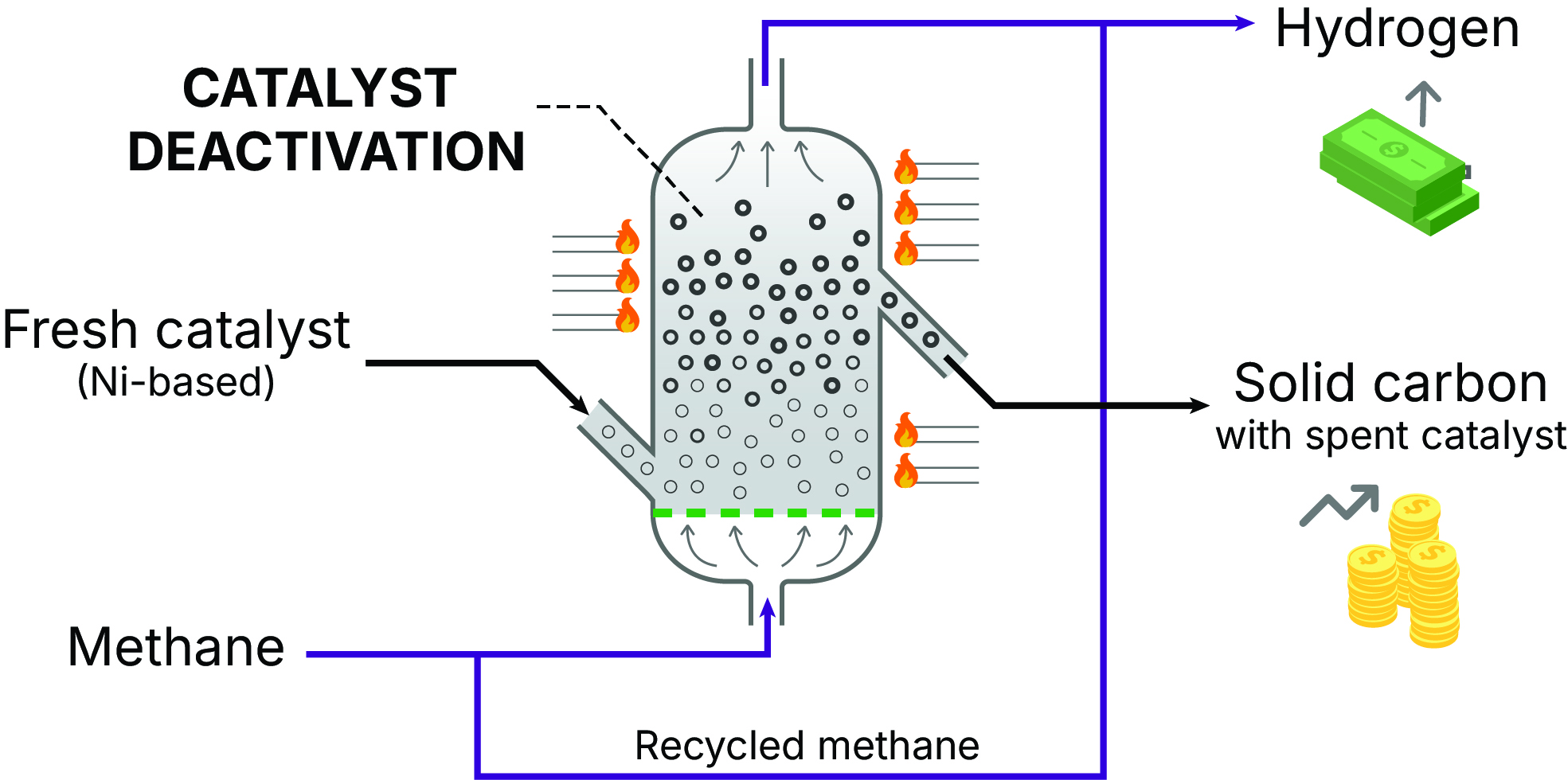
The techno-economic analysis evaluates key plant performance indicators, including capital and operating costs, the levelized cost of hydrogen (LCOH), the net present value (NPV), and the payback time (PBT). Following our baseline scenario assumptions—e.g., an H₂ production capacity of 100 TPD and a solid carbon byproduct price of $1 per kg—the LCOH of the CMP plant falls between the typical LCOH values of SMR and electrolysis with renewable energy. Based on an H₂ selling price of $5.00 per kg, the PBT and NPV indicate that the plant is economically viable.
Sensitivity analyses reveal methane price, fixed production costs, catalyst price, solid carbon sales, and disposal fees have a significant economic influence. The PBTs and NPVs of the plant at different H2 selling price and tax rates highlight the relationship between H₂ price and process viability. The sensitivity analysis of solid carbon sales indicates that they can offset H₂ production costs, potentially making CMP economically superior to steam methane reforming. However, if solid carbon byproducts remain unsold, sequestration costs could challenge CMP feasibility. Additionally, we assess temperature, catalyst hold-up, reactor deactivation, and plant capacity cost sensitivities. Optimum reactor temperature is found to be at 650 °C. Plant capacity analysis from 12.5 TPD to 800 TPD indicates substantial cost improvements due to the economies of scale and predicts a cost curve for the CMP plant.
Our study evaluates the economic and emissions impacts of different reactor heating methods. Heating via methane combustion with carbon capture achieves a low H2 cost with the lowest carbon dioxide emissions, while H2 combustion heating results in the highest cost. Electric heating powered by grid electricity leads to the highest emissions.