2025 AIChE Annual Meeting
(85c) Tailoring Tricalcium Silicate Hydration and Microstructure Using Engineered Layered Double Hydroxides with Tunable Composition and Anion Exchange Capacity
Authors
Titus Egbosiuba, Missouri University of Science and Technology
Monday Okoronkwo, Missouri University of Science and Technology
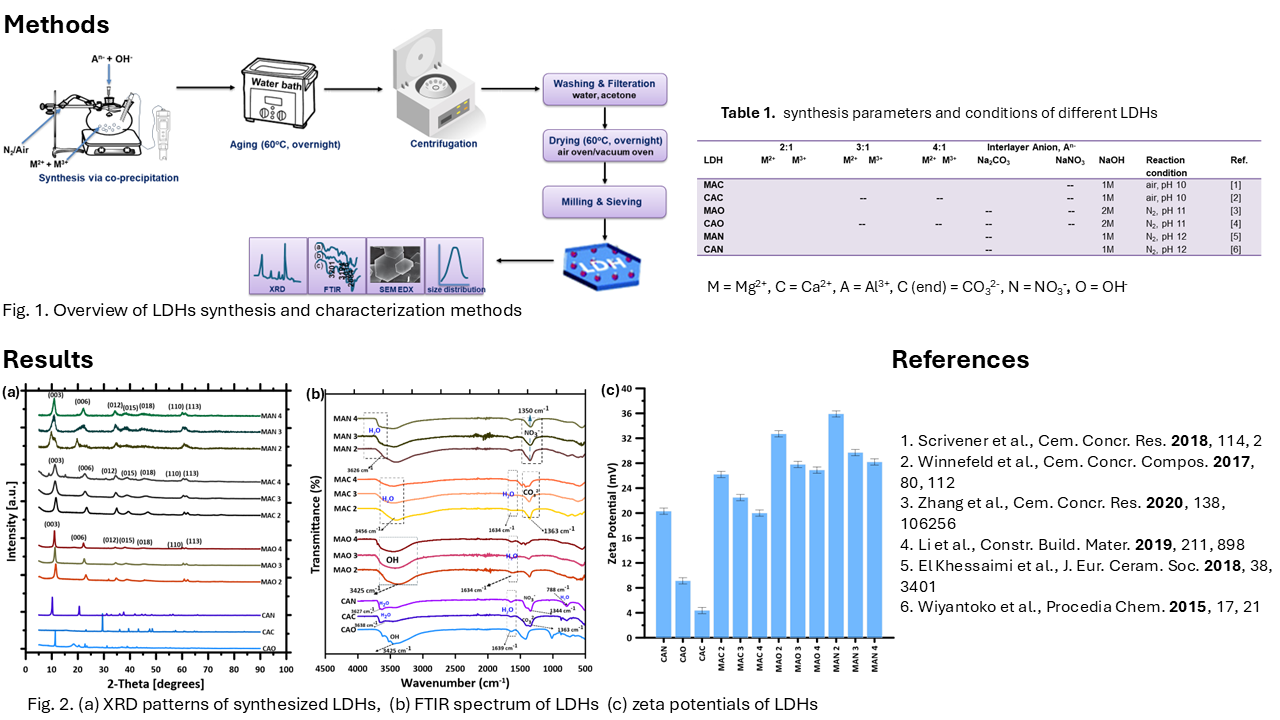
The hydration behavior and microstructural evolution of tricalcium silicate (C₃S), a principal phase in Portland cement, were systematically investigated in the presence of nano-uniform layered double hydroxides (LDHs) synthesized with varied compositions. These LDHs were designed using different divalent metals (Ca²⁺ and Mg²⁺), interlayer anions (NO₃⁻, CO₃²⁻, and SO₄²⁻), and tailored M²⁺/M³⁺ molar ratios (2, 3, and 4 in Mg-based systems). Results from isothermal calorimetry showed that the inclusion of Mg–Al LDHs with CO₃²⁻ significantly accelerated the early hydration of C₃S, advancing the main hydration peak by over 2 hours. TGA and XRD analyses confirmed increased formation of calcium-silicate-hydrate (C-S-H) and portlandite in LDH-modified systems compared to controls. Moreover, SEM images revealed a denser and more refined C-S-H morphology in samples containing Mg–Al LDHs with SO₄²⁻ interlayers, while LDHs with higher M²⁺/M³⁺ ratios promoted more uniform microstructures. These findings highlight the ability of compositionally engineered LDHs to modulate C₃S hydration kinetics, enhance microstructural development, and serve as multifunctional nano-additives for advanced cementitious materials.