2025 AIChE Annual Meeting
(303g) System-Level Analysis of Hydrogen Separation, Oxygen Removal, and Redox Materials in Solar Thermochemical Hydrogen Production
Authors
Ceria has been the state-of-the-art STCH redox material for about a decade. However, ceria has limited fuel production per cycle and requires high reduction temperatures. Other redox materials including perovskites, doped ceria, and ferrites can reduce more easily and at lower temperatures. However, these materials typically require significant excess steam during oxidation, with steam to hydrogen ratio on the order of rsteam = 102-104 [2][3], which is 1–3 orders of magnitude higher than that required for ceria. Many STCH system-level analyses assume that hydrogen separation from the dilute steam-hydrogen mixture can be done at no energetic cost by condensing steam [1][2][4]. However, this results in the loss of the latent heat of vaporization (hvap) of water during condensation: this heat cannot be reused to boil water because both processes happen at the same temperature. At the system level, this can negatively impact the overall efficiency because of the high steam demand in the oxidation step. To address this, emerging high-temperature hydrogen separation technologies, such as electrochemical separation using proton-conducting membranes, have been proposed to mitigate energy losses. However, a comprehensive thermodynamic analysis evaluating their integration with various redox materials is lacking
In this work, we develop a detailed thermodynamic model to simulate and optimize STCH hydrogen production by incorporating a range of redox materials and integrated auxiliary technologies. The model accepts redox material thermodynamic functions—specifically, enthalpy (ΔH) and entropy (ΔS) as functions of non-stoichiometry (δ)—as inputs to simulate energy and mass flow and determine hydrogen production efficiency and yield under thermodynamic equilibrium conditions. To optimize the system-level efficiency, the model adjusts key operating parameters, including reduction temperature, pressure, and oxidation extent. Ceria (CeO₂) is employed as the reference material, with comparisons drawn against alternative redox materials such as zirconia-doped ceria, metal-substituted ferrites (e.g., Ni-ferrite and Mg-ferrite), and perovskites (e.g., CTM55 [6] and LSMA [7]) reported in the literature. To expand the model’s scope, we integrate detailed modules for hydrogen and oxygen separation, each encompassing multiple technological pathways. These modules evaluate the impact of oxygen removal and hydrogen separation strategies on system efficiency for each redox material. In addition, the model accounts for variations in solid-phase heat recuperation to provide a more comprehensive assessment of system performance.
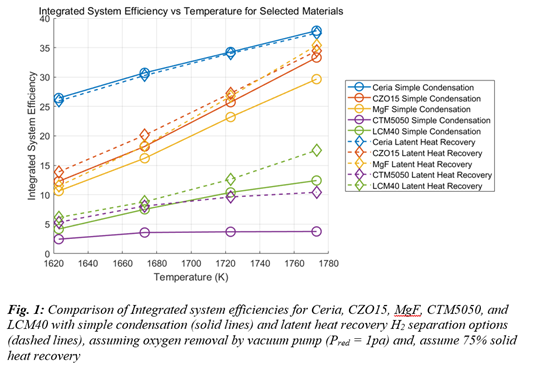
For hydrogen separation, we consider: (i) baseline condensation, (ii) high- and intermediate-temperature proton-conducting ceramic membranes (operating in the range of 200°C to 800°C), and (iii) mechanical vapor recompression systems that recover and reuse the latent heat from steam condensation. For oxygen removal, we include thermochemical oxygen pumping (TcOP) and staged mechanical vacuum pumping via a pressure cascade [5]. Preliminary simulation results as shown in Fig.1 indicate that, under reduction conditions of 1400 °C and 1 Pa, an oxidation temperature of 800 °C and ambient pressure with 75% solid heat recovery, latent heat recovery through vapor recompression effectively reduces the steam generation penalty across all redox materials in comparison to simple condensation. However, the additional work required to operate the heat pump slightly decreases the overall system efficiency for ceria—from 31.5% to 30.4%. In contrast, for LCM40, the same strategy improves system efficiency significantly, increasing it from 6.85% to 8.96%.
This work provides a comprehensive thermodynamic analysis of the influence of efficient hydrogen and oxygen separation technologies on overall STCH system performance across a variety of redox materials. Ultimately, the goal is to develop an open-source computational tool that enables the research community to screen novel redox materials and assess integrated system-level efficiency using material-specific thermodynamic data.
References
[1] L. Muhich, S. Blaser, M. C. Hoes, and A. Steinfeld, “Comparing the solar-to-fuel energy conversion efficiency of ceria and perovskite based thermochemical redox cycles for splitting H₂O and CO₂,” Int. J. Hydrogen Energy, vol. 43, no. 41, pp. 18814–18831, Oct. 2018, doi: 10.1016/j.ijhydene.2018.08.137.
[2] Li, V. M. Wheeler, A. Kumar, M. B. Venkataraman, C. L. Muhich, Y. Hao, and W. Lipiński, “Thermodynamic guiding principles for designing nonstoichiometric redox materials for solar thermochemical fuel production: Ceria, perovskites, and beyond,” Energy Technol., vol. 9, no. 5, May 2021, Art. no. 2000925, doi: 10.1002/ente.202000925.
[3] Bayon, A. de la Calle, K. K. Ghose, A. Page, and R. McNaughton, “Experimental, computational and thermodynamic studies in perovskites metal oxides for thermochemical fuel production: A review,” Int. J. Hydrogen Energy, vol. 45, no. 23, pp. 12653–12679, Apr. 2020, doi: 10.1016/j.ijhydene.2020.02.126.
[4] Lou, Z. Tian, Y. Wu, X. Li, X. Qian, S. M. Haile, and Y. Hao, “Thermodynamic assessment of nonstoichiometric oxides for solar thermochemical fuel production,” Solar Energy, vol. 241, pp. 504–514, Jul. 2022, doi: 10.1016/j.solener.2022.05.008.
[5] S. Patankar, X.-Y. Wu, W. Choi, H. L. Tuller, and A. F. Ghoniem, “A comparative analysis of integrating thermochemical oxygen pumping in water-splitting redox cycles for hydrogen production,” Solar Energy, vol. 264, Art. no. 111960, Nov. 2023, doi: 10.1016/j.solener.2023.111960.
[6] Ezbiri, M. Takacs, D. Theiler, R. Michalsky, and A. Steinfeld, “Tunable thermodynamic activity of LaₓSr₁−ₓMnᵧAl₁−ᵧO₃−δ (0 ≤ x ≤ 1, 0 ≤ y ≤ 1) perovskites for solar thermochemical fuel synthesis,” J. Mater. Chem. A, vol. 5, pp. 4172–4182, 2017, doi: 10.1039/C6TA06644E.
[7] Qian, J. He, E. Mastronardo, B. Baldassarri, W. Yuan, C. Wolverton, and S. M. Haile, “Outstanding properties and performance of CaTi₀.₅Mn₀.₅O₃–δ for solar-driven thermochemical hydrogen production,” Matter, vol. 4, no. 2, pp. 688–708, Feb. 2021, doi: 10.1016/j.matt.2020.11.016.