2025 AIChE Annual Meeting
(51a) Synergistic Adsorption and Catalysis for CO2 Storage and Its Methanation (CSM) over Ni–K2CO3 Supported on Al2O3
Authors
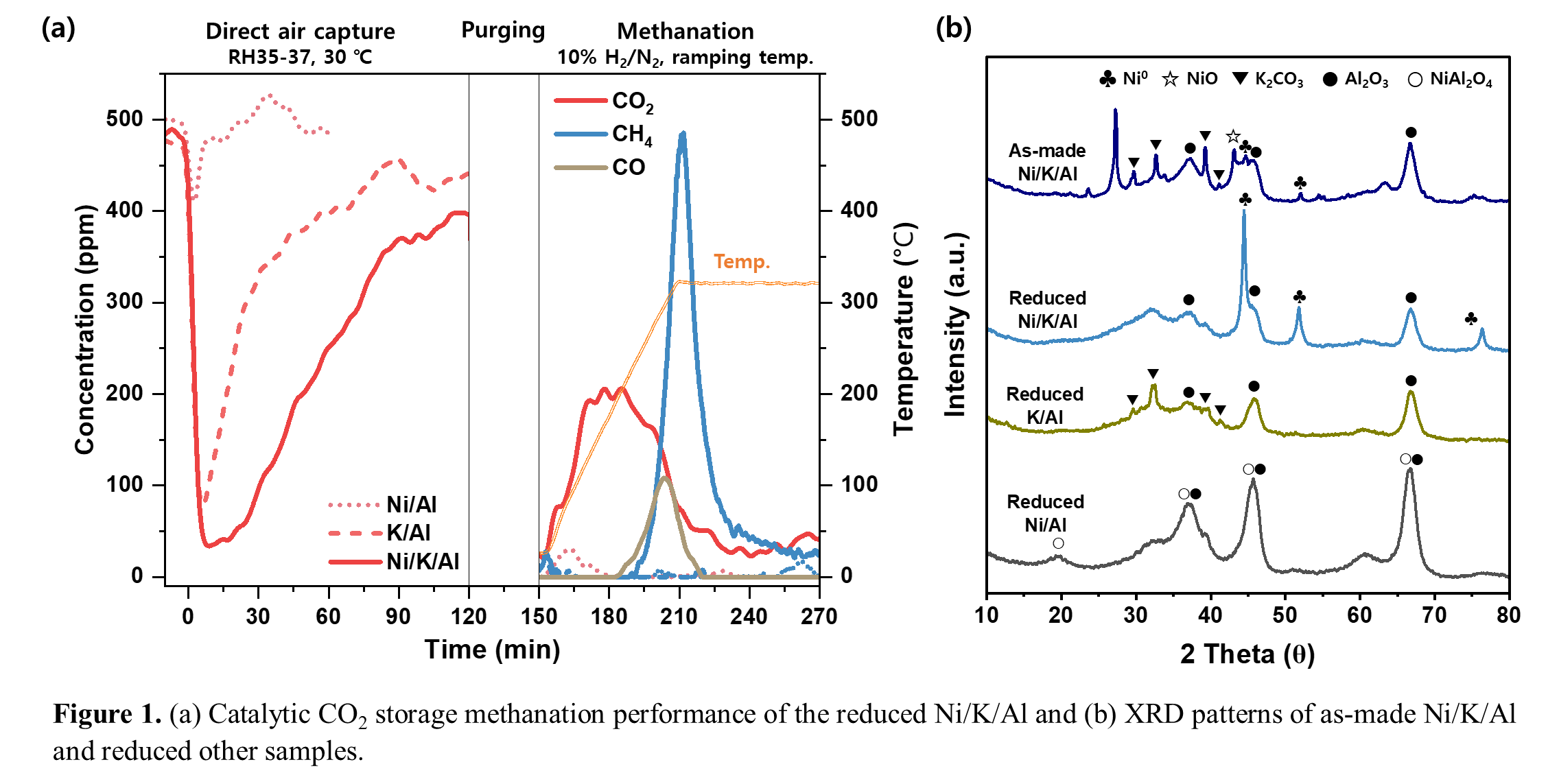
Figure 1a demonstrates that the Ni-K2CO3/Al2O3 (Ni/K/Al) effectively operated in the CSM system. In addition, Ni/K/Al achieved a CO2 capacity of 2.35 mmol/g, nearly three times higher than K2CO3/Al2O3 (K/Al). These results indicate that Ni is not only active for methanation but also promotes the generation of additional CO2 adsorption sites. This synergistic enhancement for adsorption was attributed to the reductive decomposition of K2CO3 during H2 pretreatment, forming KO‒ superbase sites that enhance CO2 adsorption.
XRD analysis (Figure 1b) confirmed K2CO3 decomposition in Ni/K/Al after H2 pretreatment, as its peaks disappeared—unlike in K/Al where they remained—supporting the proposed mechanism. Additionally, a sharp metallic Ni peak appeared in Ni/K/Al, while Ni in Ni/Al formed inactive NiAl2O4 spinel, making it ineffective for methanation. These results suggest that K2CO3 suppresses strong Ni– Al2O3 interactions, preventing NiAl2O4 formation and promoting reducible Ni oxide dispersion on K2CO3, enhancing methanation activity.
This study demonstrated the critical role of interfacial interactions between Ni and K2CO3 in enabling efficient CSM. Ni facilitated the in-situ generation of active CO2 adsorption sites through K2CO3 decomposition, while K2CO3 prevented NiAl2O4 formation and promoted Ni dispersion, which further confirmed the catalytic advantages of the Ni/K/Al system. We will discuss in greater detail during the presentation, along with additional performance data, characterization and reaction mechanisms for CMS at the upcoming AIChE meeting.