2025 AIChE Annual Meeting
(354b) Sustainable Synthesis of Ultrathin Non-Van Der Waals 2D Uio-66-NH? Films for High-Performance Ion Separation
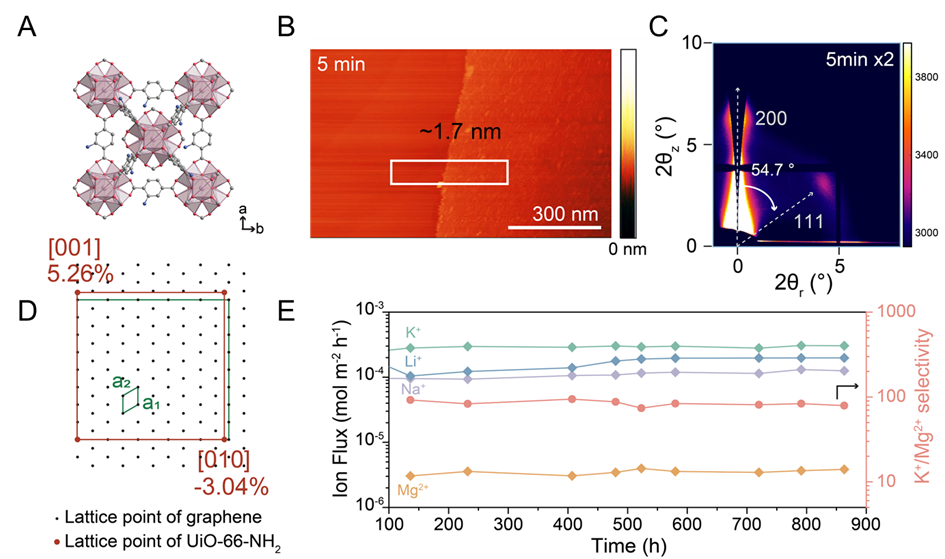
Characterization by AFM, XPS, and GIWAXS confirmed sub-10 nm pinhole-free films with high crystallinity and preferential orientation (Figure 1C and D). These films demonstrated exceptional monovalent/divalent ion selectivity (e.g., K⁺/Mg²+ selectivity up to 99.8) via size exclusion and electrostatic interactions, attributed to linker defects and amino-functionalized pores. Stability tests revealed over 36 days of performance in 0.1 M salt solutions, underscoring their robustness (Figure 1E). The synthesis method-free of organic solvents, high temperatures, and prolonged reaction times-aligns with green chemistry principles and offers a pathway to ultrathin MOF membranes for energy-efficient separations.
This work advances the field of non-vdW 2D materials, providing a scalable platform to engineer MOF films for applications in desalination, ion recovery, and selective molecular transport, with significant implications for sustainable chemical processes.
References
[1] A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke, P. Behrens, Chemistry – A European Journal 2011, 17, 6643-6651.
[2] H.-Y. Chi, S. Song, K. Zhao, K.-J. Hsu, Q. Liu, Y. Shen, A. F. Sido Belin, A. Allaire, R. Goswami, W. L. Queen, K. V. Agrawal, J. Am. Chem. Soc. 2025.