2025 AIChE Annual Meeting
(399n) Sugarcane Bagasse Hydrochar Modified with Citric Acid. Sustainable Biomaterial for Removing Heavy Metals from Aqueous Solutions
Authors
Sugar cane (Saccharum spp.) is the most widely cultivated crop globally, responsible for approximately 75% of the world's sugar production, and Mexico is one of the major producers. In this context, sugar cane bagasse, a lignocellulosic by-product generated in large volumes, has attracted increasing interest as an adsorbent material in wastewater treatment. Its application for the removal of emerging pollutants, heavy metals and dyes represents an efficient and cost-effective solution, and it contributes to the revalorization of this agro-industrial waste and the mitigation of environmental pollution.
Water contamination by heavy metals, particularly cadmium (Cd2+), poses a substantial threat to ecosystems and human health. Cadmium (Cd) is classified as a class I carcinogen, exhibiting high toxicity even at low concentrations, with documented adverse effects including nephrotoxicity, reproductive dysfunction, hematological disorders and cardiovascular conditions. Its accumulation in living organisms occurs primarily through the consumption of contaminated foodstuffs, with its main sources of emission including industrial, mining and agricultural activities. The high mobility of Cd(II) in the aquatic environment, coupled with its strong affinity for adsorption in organic matrices, renders it one of the most concerning metals in terms of environmental pollution.
In this study, the efficiency of cane bagasse (CB) was modified by hydrothermal treatment (CBHC). The procedure for obtaining this biochar from agro-industrial waste is documented in technical literature. In addition, the CB and CBHC were functionalized employing a hydrothermal procedure utilizing different citric acid (CA) concentrations of 0.5, 1 and 2 M, aiming to incorporate functional groups, mainly phenolic and carboxylic sites, to improve the adsorption capacity of the material towards Cd(II) in aqueous solutions. Various materials were synthesized to find the optimal conditions in this process, and it was found that hydrocarbonized sugar cane bagasse modified with 1 M citric acid (CBHC-CA1) presented the highest adsorption capacity. This enhanced capacity was attributed to incorporating acidic functional groups, particularly carboxylic and phenolic, on the surface of the CBHC following modification with CA.
The CBHC-CA1 material was characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), nitrogen physisorption and zeta potential, and the acidic and basic sites concentrations were determined by an acid-base titration method. Furthermore, a comprehensive investigation was undertaken to study the effects of pH, temperature, ionic strength and reversibility on the adsorption capacity and mechanisms. The results indicated that Cd(II) adsorption is predominantly due to electrostatic interactions between the deprotonated sites of the carboxylic groups and the Cd2+ ions. At a pH of 7 and a citric acid concentration of 1 M, an adsorption capacity of 100 mg/g was attained. This adsorption capacity is higher than that of conventional materials like clays, zeolites, activated carbons, modified activated carbons and natural biosorbents such as CB and wood sawdust.
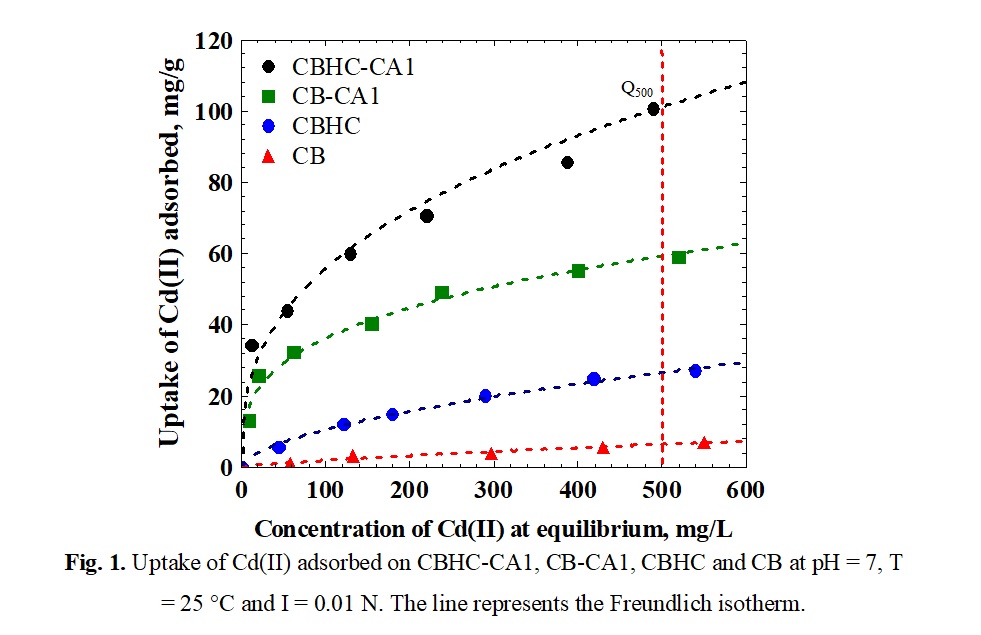
The adsorption isotherms of Cd(II) on CB, CBHC, CB-CA1 and CBHC-CA1 are depicted in Figure 1. It can be noted that the decreasing order of the adsorption capacities is as follows: CBHC-CA1, CB-CA1, CBHC and CB. The adsorption capacities evaluated at a Cd(II) equilibrium concentration of 500 mg/L and designated as Q500 are 1000, 59, 27 and 7 mg/g for CBHC-CA1, CB-CA1, CBHC and CB. The adsorption capacity of CBHC-CA1 is about 14 times higher than that of CB. Hence, the carbonization and functionalization of CB considerably increased its adsorption capacity towards Cd(II).
The study of the effect of pH revealed that increasing pH favors the capacity of CBHC-CA1 for adsorbing Cd(II), which is explained by the higher negatively charged surface of the material under alkaline conditions. FT-IR characterization confirmed the presence of spectroscopic bands associated with carboxylic and phenolic groups incorporated on the material surface during modification using the CA solution. The concentration of active sites was determined to be predominantly acidic, with a total concentration of 4.75 meq/g for acidic sites and 2.5 meq/g for carboxylic sites. Finally, it was demonstrated that the adsorption of Cd(II) on CBHC-CA1 is a partially reversible and endothermic process.
In conclusion, the adsorption capacity of sugarcane bagasse for Cd(II) increased after the hydrothermal treatment using a citric acid modification (CBHC-CA1). The optimal conditions were 1 M citric acid at pH 7, achieving a maximum adsorption capacity of 100 mg/g for CB-HC-CA1. Carboxylic acid sites played a key role in the adsorption mechanism, and higher pH enhances Cd(II) adsorption. The effect of the ionic strength on the adsorption capacity confirmed that electrostatic attractions are critical for enhancing the adsorption, while thermodynamic studies indicated an endothermic and partially reversible process involving both physical and chemical mechanisms. CBHC-CA1 is a cost-effective and promising adsorbent for heavy metal removal. These findings show the potential of applying the modified sugarcane bagasse as an efficient and cost-effective adsorbent for removing heavy metals from contaminated waters, thus providing a novel and viable strategy for the sustainable management of agro-industrial wastes and the mitigation of environmental pollution.