2025 AIChE Annual Meeting
(695d) Submicron Films of Polymer Blends: Unlocking Macrophase Separation to Enhance Membrane Gas Separation Properties
Authors
Narjes Esmaeili - Presenter, University at Buffalo, The State University of New York
Leiqing Hu, University At Buffalo
Erda Deng, University At Buffalo
Vinh Bui, University at Buffalo
Haiqing Lin, University of Buffalo, State University of New Yor
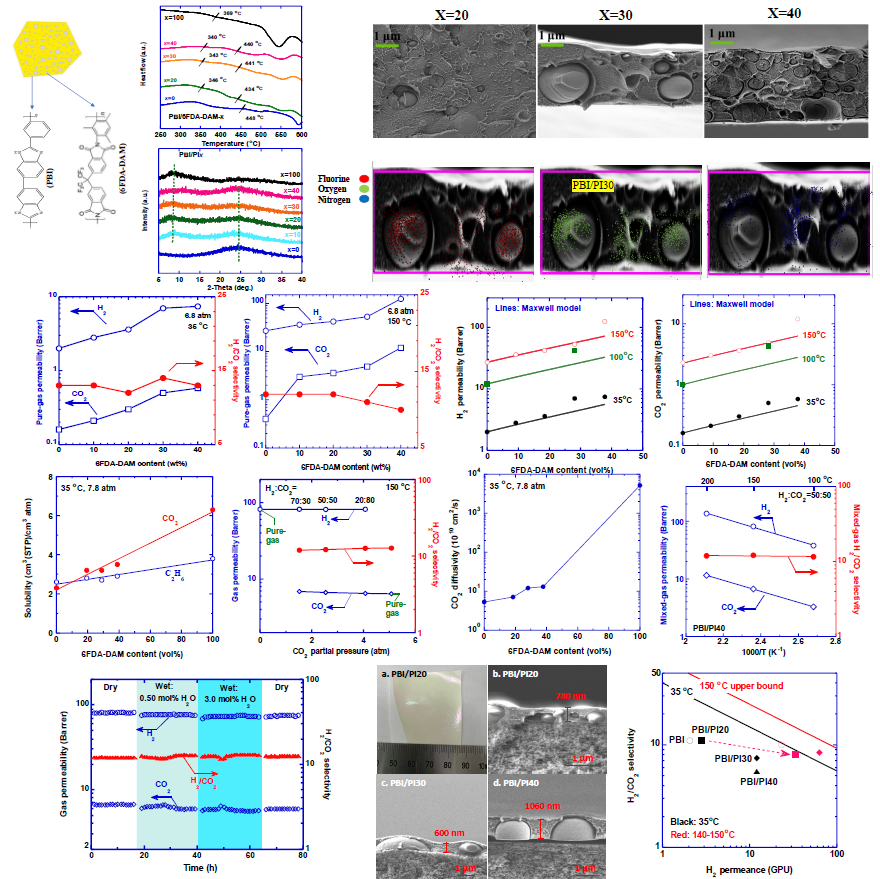
High-performance polymeric materials are essential to enable energy-efficient membrane technology in hydrogen purification and carbon capture. However, polymers face the permeability/selectivity trade-off, and they can be incorporated with inorganic fillers to improve gas transport properties; however, the two distinctive phases often have interfacial incompatibility, making them difficult to fabricate into defect-free submicron thin-film composite (TFC) membranes. In this study, we demonstrate macrophase-separated polymer blends with superior H2/CO2 separation properties by dispersing a highly permeable and non-selective polyimide (6FDA-DAM, PI) phase in a highly selective but moderately permeable continuous polybenzimidazole (PBI) phase. At loadings of 10-40 wt%, PI forms a macroscopic discontinuous phase in the blends, as confirmed by scanning electron microscope (SEM) images, two distinct d-spacings from WAXD patterns, and two glass transition temperatures in DSC curves. The effect of the PI loading on H2 permeability can be satisfactorily described using the Maxwell model. For instance, adding 40 mass% PI in PBI increases H2 permeability from 27 to 120 Barrer with H2/CO2 selectivity of 10 at 150 °C, breaking the traditional trade-off and surpassing the upper bound. More importantly, the blends can be fabricated into TFC membranes while retaining their macrophase separation, leading to a significant increase in H2 permeance compared to PBI-based membranes. For example, adding 30% PI increases H2 permeance by 250%, from 18 to 63 GPU, while slightly decreasing H2/CO2 selectivity from 10 to 8.4 at 140 ℃. This facile and elegant approach of polymer blending provides a new platform for designing high-performance membranes for gas and liquid separations with great manufacturability.