2025 AIChE Annual Meeting
(445e) Study of (Semi-)Batch Reactive Crystallization of L-Glutamic Acid Using PAT, Focusing on Polymorphism and Crystal Habit
Authors
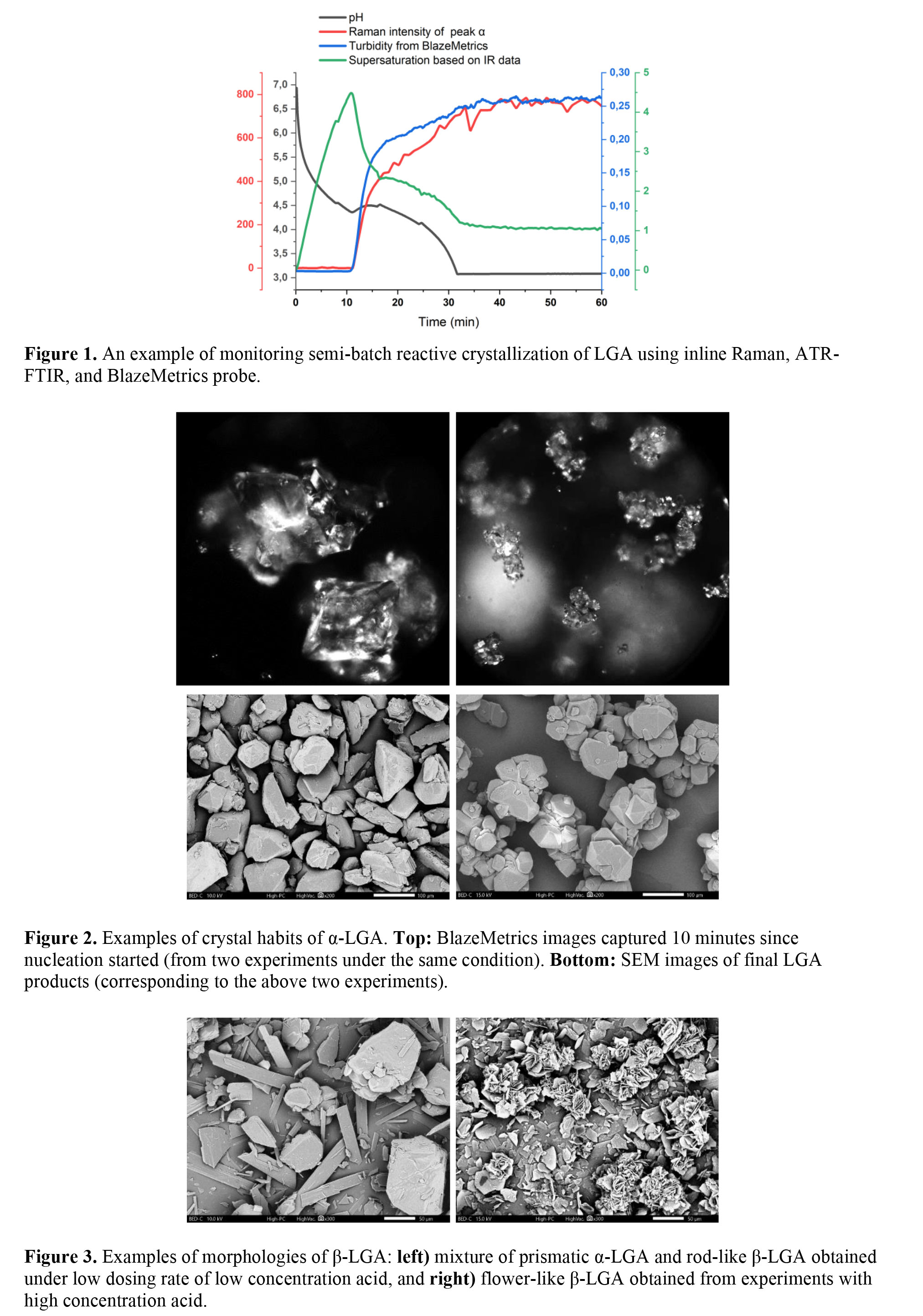
Materials & Methods The selected compound L-glutamic acid (LGA) has two polymorphic forms with different crystal shapes, the metastable α-form of prismatic shape and the stable β-form of needle shape and spherulites [4][5]. An un-seeded reactive crystallization study of LGA was performed by mixing sodium glutamate solution with equimolar sulfuric acid. The temperature was maintained at 25 °C. Different dosing rates of acid, including batch instant addition and semi-batch continuous addition, and different concentrations of reactants were applied to build different supersaturation generation profiles. Process analytical technology (PAT) tools were employed to monitor the reaction and crystallization in real time. Supersaturation levels were monitored by ATR-FTIR, while turbidity and particle imaging information were captured by the BlazeMetrics in-situ microscope. Polymorphic information was tracked by inline Raman spectroscopy. Figure 1 gives an example of real-time measurement during a 30-minute semi-batch reactive crystallization followed by a 30-minute holding period. Additionally, final crystal properties such as polymorphism, morphology, and particle size were analyzed offline.
Results Batch crystallization experiments that involved dosing all acid at once produced α-LGA at low supersaturation and mixture of α-LGA and β-LGA at high supersaturation, which reflects the kinetic advantage of α-LGA, consistent with the findings in the literature[4]. However, the polymorphism in the semi-batch experiments seemed to be more controlled than batch ones. Nearly pure α-LGA was obtained under different dosing rates, while there were exceptions under very slow dosing rates. The mixture of the α- and β-forms was obtained perhaps due to polymorph transformation during the long dosing period. The higher concentration of acid resulted in the mixture of the α- and β-forms, probably because of β-LGA nucleating at relatively high local supersaturation. The crystal habit was studied by both inline images from BlazeMetrics probe and offline images from SEM. The nucleation point was determined based on turbidity. Among the semi-batch experiments, under a constant acid dosing rate producing α-LGA, the supersaturation level at the nucleation point was found to influence the crystal habit. Inline imaging data indicates two distinct particle morphologies of α-LGA, either large single crystals with less agglomeration or agglomerates of small crystals, which are confirmed by SEM images (Figure 2). The experiments producing agglomerates of fines have three times higher turbidity level, smaller average median length and more particle numbers than the experiments producing large single crystals while the solid yields are close. Among the experiments producing the mixture of α- and β-forms, two different morphologies of β-LGA - either rod-like or flower-like - were obtained (Figure 3). In addition, the crystallization yields obtained under the same concentration of acid and different dosing rates were similar, while higher acid concentrations resulted in higher yields due to lower LGA loss in mother liquor.
Conclusions & Ongoing work In this study, reactive crystallization of LGA was conducted under different supersaturation generation profiles. It was found that both the supersaturation levels at the nucleation point and the supersaturation generation rates affect the competition between nucleation, growth and agglomeration kinetics. The comparison between batch and semi-batch experiments reveals that generating supersaturation rapidly or building up supersaturation gradually may lead to distinct differences in nucleation behavior, crystal growth, polymorphism, and product quality. Ongoing research is investigating ultrasound’s potential to mitigate the stochasticity of the nucleation point, thereby enabling better control over final crystal properties. These findings address the role of supersaturation generation and impact of the nucleation point in reactive crystallization processes and may offer potential insights for process optimization.
Reference
[1] J. Jordens, B. Gielen, C. Xiouras, M.N. Hussain, G.D. Stefanidis, L.C.J. Thomassen, L. Braeken, T. Van Gerven, Sonocrystallisation: Observations, theories and guidelines, Chem. Eng. Process. - Process Intensif. 139 (2019) 130–154. https://doi.org/10.1016/j.cep.2019.03.017.
[2] B. Zhang, I. Ådnebergli, G.D. Stefanidis, T. Van Gerven, Effects of Ultrasound on Reactive Crystallization and Particle Properties of an Aromatic Amine in Batch and Continuous modes, Ultrason. Sonochem. 111 (2024) 107121. https://doi.org/10.1016/j.ultsonch.2024.107121.
[3] B. Zhang, G.D. Stefanidis, T. Van Gerven, Can Ultrasound Replace Seeding in Flow Reactive Crystallization of an Aromatic Amine?, Org. Process Res. Dev. (2024). https://doi.org/10.1021/acs.oprd.4c00385.
[4] R. Achermann, A. Košir, B. Bodák, L. Bosetti, M. Mazzotti, Process Performance and Operational Challenges in Continuous Crystallization: A Study of the Polymorphs of L-Glutamic Acid, Cryst. Growth Des. 23 (2023) 2485–2503. https://doi.org/10.1021/acs.cgd.2c01424.
[5] C.P.M. Roelands, J.H. ter Horst, H.J.M. Kramer, P.J. Jansens, Precipitation mechanism of stable and metastable polymorphs of L‐glutamic acid, AIChE J. 53 (2007) 354–362. https://doi.org/10.1002/aic.11072.