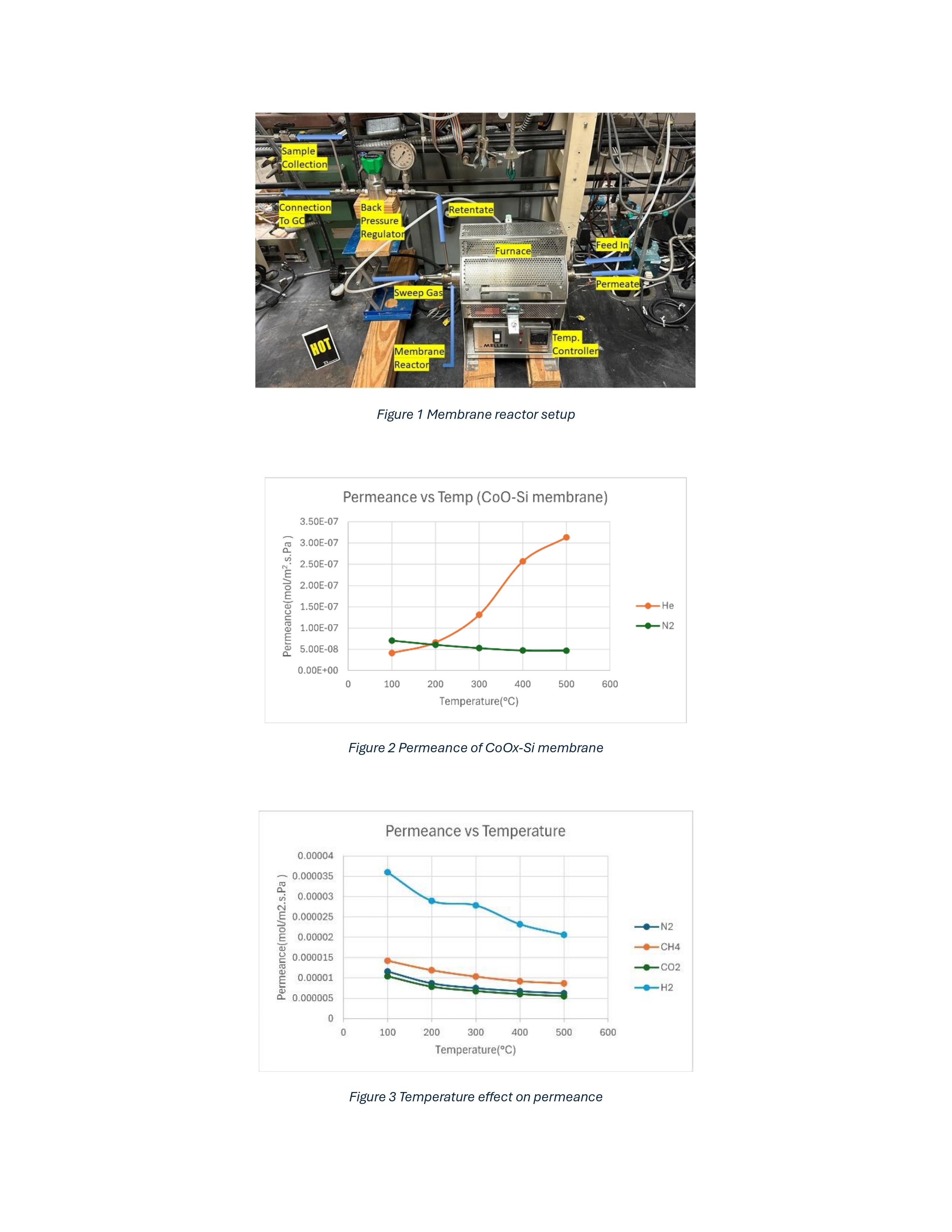
2025 AIChE Annual Meeting
(446d) Structured Catalytic Membrane Reactor for Sustainable Hydrogen from Natural Gas Reforming
Previous works have shown that silica membranes can work well for gas separation but are susceptible to densification in the presence of steam at elevated temperatures.1 Literature data shows that addition of metal oxides in the silica layer enhances hydrothermal stability while enabling activated, selective transport of hydrogen at high temperatures.2 When combined with a hydrogen generating reaction such as SMR or water gas shift (WGS) conversion may exceed beyond thermodynamic values. Herein we report on progress with cobalt oxide-silica-coated alumina membrane synthesis and characterization as first step towards development of the membrane reformer.
Porous α-alumina tubes (3 mm i.d. and 5 mm o.d.) are used as the substrate. A γ-alumina layer is deposited on this porous support by the slipcasting method using boehmite solutions. For preparation of the permselective layer, the sol-gel dip coating method is used using a mixture of Tetraethoxysilane (TEOS) and cobalt nitrate hexahydrate. The mixture is added drop-wise to achieve a final molar ratio of 255 [EtOH]:4 [TEOS]:1 [Co(NO3)2.6H2O]:9 [H2O2]:40 [H2O]. All the sols remained clear and transparent during the sol synthesis, which indicated that the sols were stable and homogenous. Earlier prepared asymmetric tubes were dip-coated in this sol, and then the membrane tubes and sol were dried in an oven at 65 °C for 110 h. After drying, the fibers were calcined at 600 °C for 4 hours at a ramp rate of 0.7 °C/min in a muffle furnace.
The prepared membranes are used in the reactor setup as shown in Fig. 1. The gas flow is metered through mass flow controllers to the reactor setup, and a back pressure regulator is connected to the retentate side to maintain the system pressure. The membrane ends are sealed for gas tight seal with ceramic glaze and connected to the stainless-steel tubes through graphite ferrule to develop a leak-proof seal. The gas flows are measured at the respective permeate and retentate ends through bubble gas flowmeters, and their respective concentrations can be measured through a gas chromatograph.
A first set of CoOx-Si membranes were prepared and tested for helium and nitrogen permeance. Helium has a similar kinetic diameter to H2 but is non-adsorbing. This data shows an increase in the He permeance with increasing temperature (Fig. 2), indicating temperature activated transport.
Single gas permeation studies were conducted to check the permeability of the gases through the CoOx-Si layer. A nearly constant permeance vs. average total pressure rules out leaks or convection through larger defects. Fig. 3 shows a decrease in gas permeance, as expected, except for H2. This decreasing trend and the rank-ordering of gases with molecular weight suggest that the membrane morphology enables Knudsen diffusion. Membrane synthesis and permeation experiments are underway to determine reproducible methods for preparing H2-permselective silica membranes. We are currently investigating different calcination temperatures and multiple dipping and drying steps to ensure that the permselective layer is uniform and pinhole-free, which helps with the activated transport of H2.
We will present results with coupled H2 generation and removal using CoOx-Si membranes in a SMR reactor. The next steps are to test the effect of different metal oxides and effect of their concentration for increased hydrothermal stability of the membrane while maintaining a high flux of H2 with significant purity during the WGS and SMR reactions.
References: -
- Boffa, V., Blank, D. H. A. & ten Elshof, J. E. Hydrothermal stability of microporous silica and niobia-silica membranes. J Memb Sci 319, 256–263 (2008).
- Uhlmann, D., Smart, S. & Diniz Da Costa, J. C. High temperature steam investigation of cobalt oxide silica membranes for gas separation. Sep Purif Technol 76, 171–178 (2010).