2025 AIChE Annual Meeting
(696i) Structure-Transport Relationships in Crosslinked Redox-Active Polymer Networks for Energy Storage
Authors
Juan De Pablo, University of Wisconsin-Madison
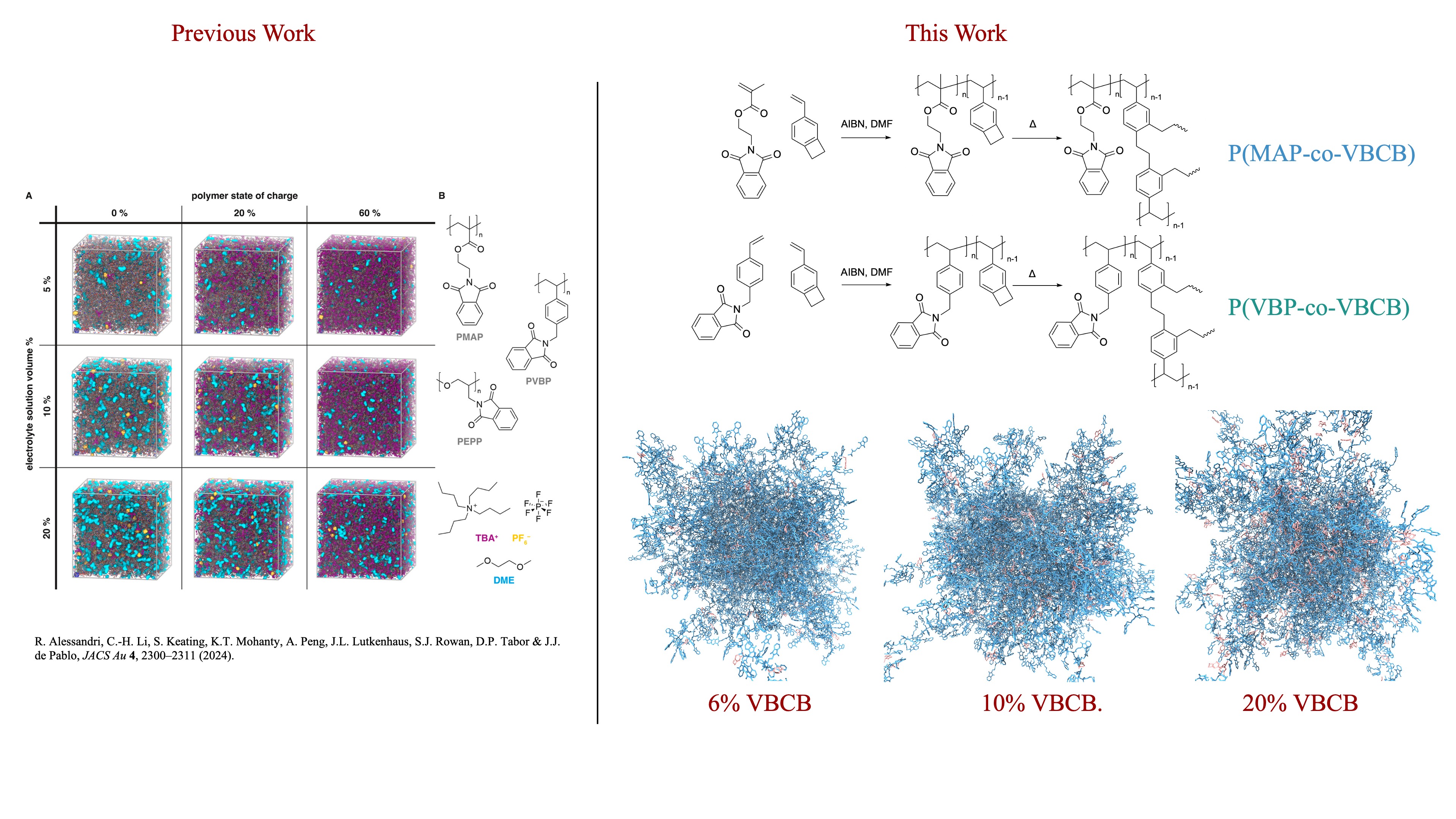
Redox-active polymers are promising candidates for sustainable, high-capacity electrode materials in next-generation organic batteries. Their tunable redox behavior, synthetic versatility, and environmentally friendly composition offer advantages over conventional inorganic systems. However, a major drawback of linear polymer architectures is their tendency to dissolve in liquid electrolytes during cycling, leading to capacity loss and a shortened device lifespan. To address this challenge, experimental efforts have developed crosslinked redox-active copolymer networks—composed of redox-active units and covalent crosslinkers—that form stable, insoluble, three-dimensional structures and maintain structural integrity in electrolyte environments. Building on our previous work in atomistic modeling of redox-active homopolymers, this study employs large-scale molecular dynamics simulations to investigate structure–property relationships in these crosslinked polymer networks. We systematically vary two key design parameters: (1) the degree of crosslinkers and (2) the electrolyte solution content. Our simulations characterize the spatial distribution of redox-active domains, quantify electronic percolation, and examine the impact of crosslinking density on polymer segmental dynamics and ionic mobility. Our results reveal that moderate crosslinking strikes a favorable balance between mechanical stability and ion transport, while excessive crosslinking restricts diffusion and reduces dynamic flexibility. The relationships uncovered between chemical structure, morphology, and transport properties provide valuable insights for designing redox-active polymers with enhanced performance in all-organic battery systems.