2025 AIChE Annual Meeting
(584cw) Structure Property Relationship of Pt Cluster Catalysts for Propane Dehydrogenation
Authors
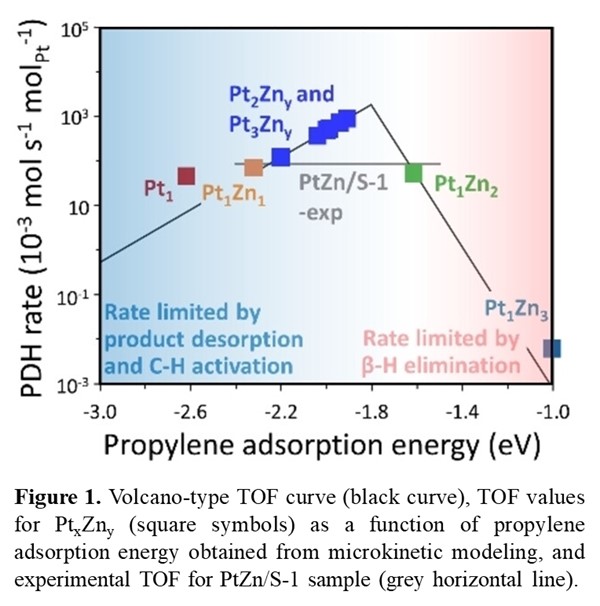
We find that the coordination of a Pt atom to a nest of grafted Zn atoms increases the stability of the Pt1Zny sites, whose activity is similar for y=0–2 and drops dramatically for y>2. We further demonstrate, via linear scaling relations and MKM, that the turnover frequency obeys a volcano law as a function of propylene binding strength (Figure 1). The Pt2Zn1 and Pt3Zn1 sites are stable and exhibit activity similar to Pt1Zn2, but only Pt1Zn2 manifests reaction kinetics consistent with experimental data, strongly suggesting the active site composition in the synthesized catalyst samples. The methodology presented here also suggests a general strategy for deducing active site information such as composition through simple kinetic experiments. In addition, propylene binding strength has been found as a universal descriptor for propane dehydrogenation activity and is used to predict activity for other Pt cluster catalysts.