2025 AIChE Annual Meeting
(429f) Structure Dependent Ionic Conductivity in Poly(ionic liquid)-Based Hybrid Electrolytes
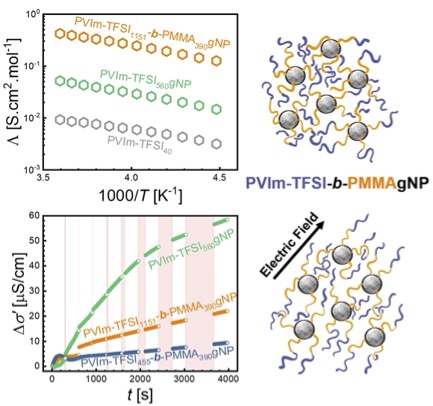
Dispersion and self-assembly of polymer-grafted nanoparticles have been utilized for diverse applications requiring enhanced thermo-mechanical properties, solute transport in membrane separations, or enhanced conductivity in hybrid electrolytes. The interfacial resistance to ion transport between the filler and polymer generally limits the conductivity in polymer nanocomposites. We aim to overcome this limitation by designing hybrid electrolytes based on single-ion conducting polymers grafted on nanoparticles. I will present how chain length and assembled structures are used to explain ion conductivity compared to that of particle-free poly(ionic liquid) homopolymer. In addition, I will present the new poly(ionic liquid)-b-poly(methyl methacrylate) (PIL-b-PMMA) copolymer-grafted system. Our results indicate that the copolymer hybrid design achieves significantly higher molar conductivity than PIL-grafted nanoparticles. The PIL block length, diblock sequence and the ionic liquid addition into copolymer morphologies change the net repulsion between nanoparticles causing pathways of different morphologies and thicknesses for ionic conduction. Lastly, I will present the results on polarizability of chains with the application of electric fields.