2025 AIChE Annual Meeting
(407e) Structure-Activity Relationships of Metal Oxide/Cu-Based Catalysts for CO2 Hydrogenation to Methanol
Authors
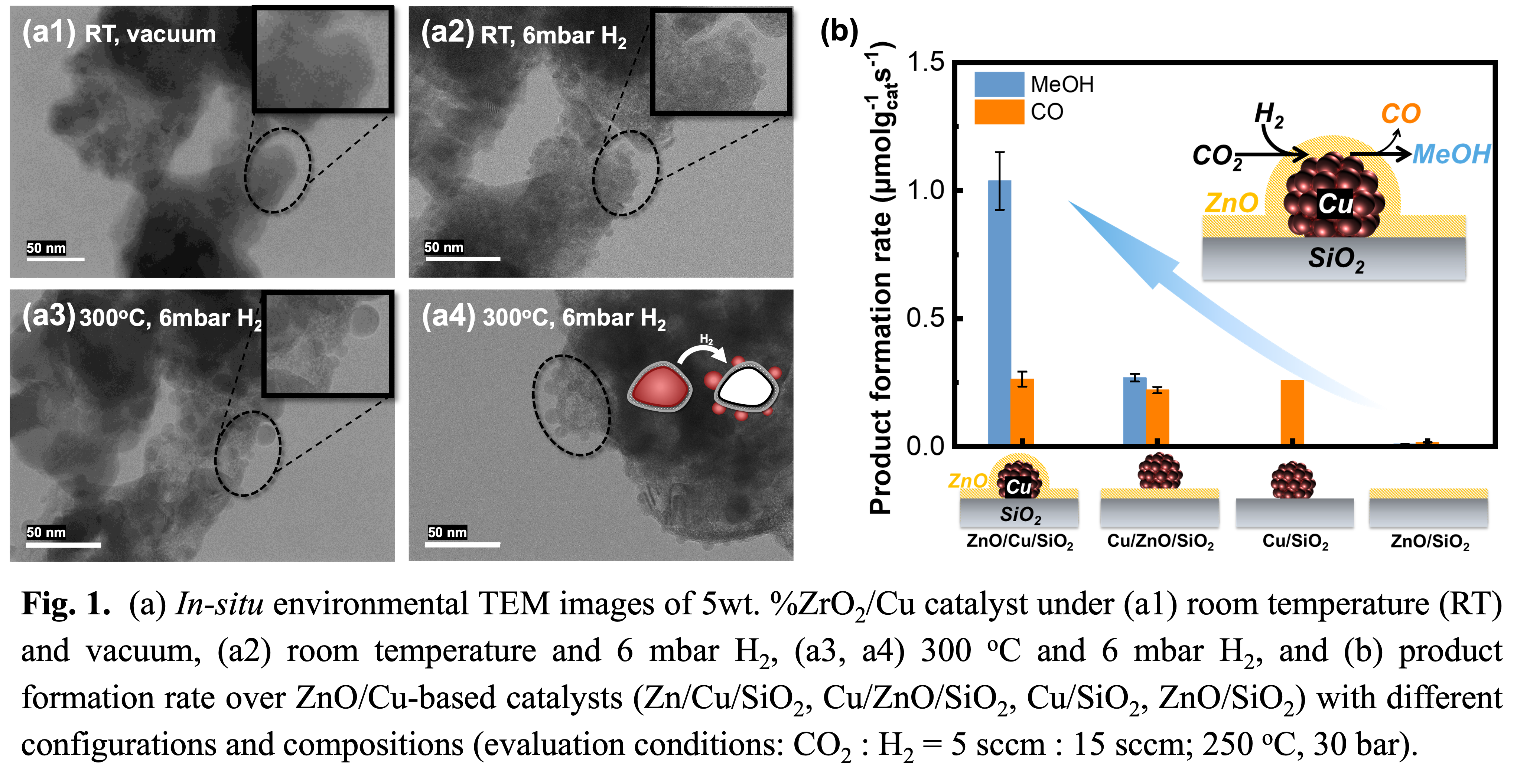
In this work, we showcase two model catalyst systems to investigate the structure-activity relationships in CO2 hydrogenation reaction: (1) inverse ZrO2/Cu (Cu particle size: 20-40 nm) synthesized via a sol-gel method, and (2) ZnO/Cu/SiO2 with uniform Cu nanocrystals (~7nm) made by colloidal chemistry and controllable ZnO layers prepared by atomic layer deposition (ALD)2). In both systems, metal oxides significantly enhance the methanol formation rates compared to bare Cu catalysts, which predominantly yield CO. For the inverse ZrO2/Cu catalysts, we identified that under reductive conditions, Cu species beneath the ZrO2 overlayer are highly dynamic, migrating to the outer surface of the ZrO2 overlayer and forming Cu nanoclusters. This restructuring behavior was tracked using Environmental TEM (Fig. 1a). In the ZnO/Cu/SiO2 system, we found that encapsulating Cu/SiO2 by ZnO ALD layers enhanced both CO2 conversion and methanol selectivity compared to Cu deposited on ZnO-modified SiO2 (Fig. 1b). In particular, the methanol selectivity increased from ~50% to ~80%, suggesting the original configuration of ZnO/Cu plays a key role in catalytic performance. In-situ DRIFTS and DFT calculation suggest that CO2 hydrogenation to methanol over both catalyst systems likely proceeds via the formate reaction pathway with CO2 activates on the Cu-metal oxide interface, and H2 activation on the surface of Cu facilitating the further hydrogenation of carbonate/bicarbonate.
- Chem. Rev. 2024, 124, 8, 4543–4678.
- ChemCatChem 2021, 13, 770-781.