2025 AIChE Annual Meeting
(198d) Structural Pair Sites for Highly Active Hydrogenation of Nitriles
Author
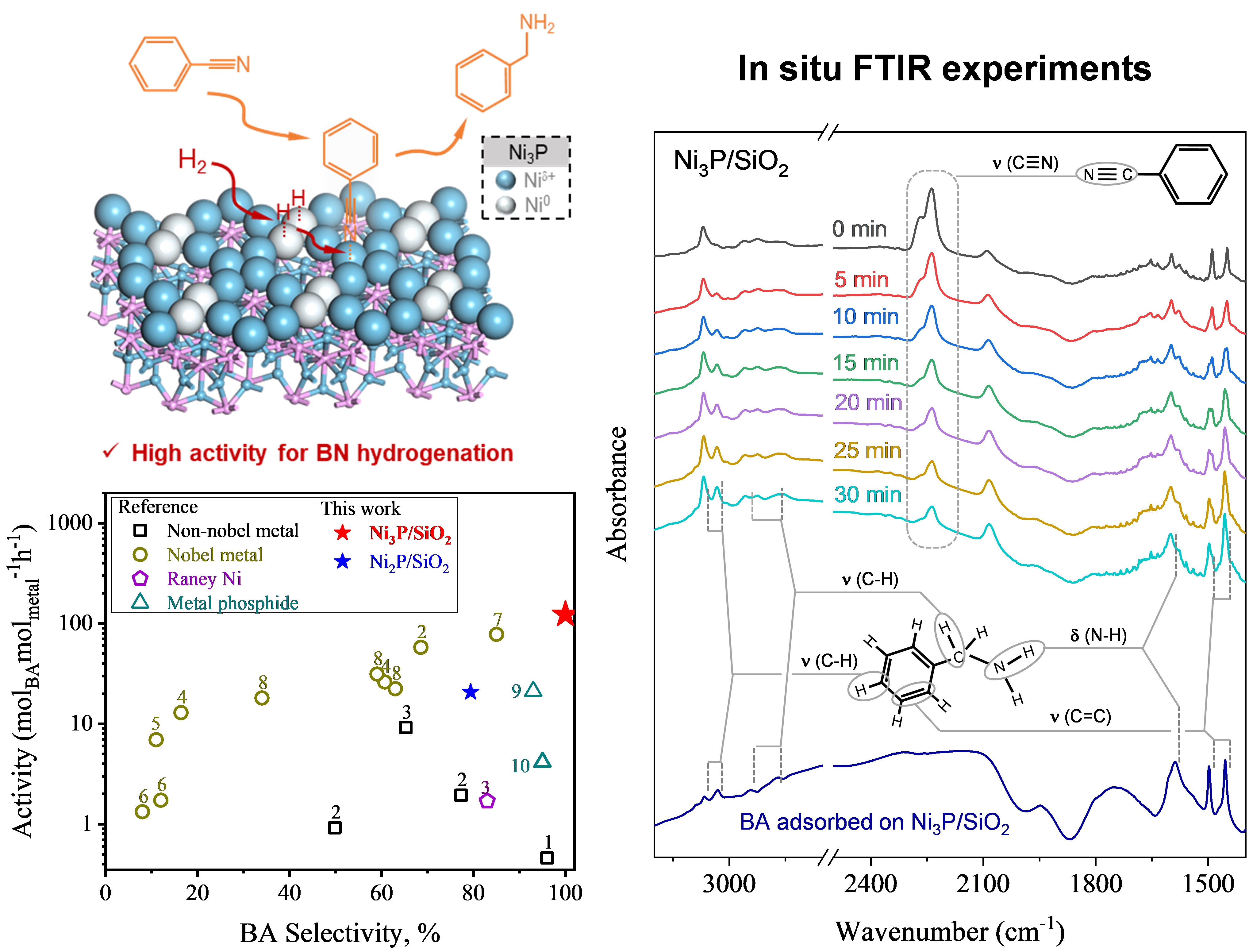
Here, we present a novel strategy for constructing metal pair-sites with heterogeneous centers, Ni0 and Niδ⁺, within the crystal phase of metal-rich Ni₃P. These Ni0-Niδ⁺ pair-sites exhibit a specific distance of 4–5 Å, creating a cooperative effect that enhances catalytic performance in hydrogenation reactions. Using benzonitrile hydrogenation as a model, we demonstrate the high efficiency of these pair-sites in selectively producing primary amines. Compared to monofunctional Ni and Ni₂P catalysts, Ni₃P shows superior activity, attributed to its well-defined Ni0-Niδ⁺ sites. Our integrated reaction experiments, in situ FTIR analysis, and DFT calculations reveal the distinct roles of each site: Ni0 activates H₂, while Niδ⁺ adsorbs and activates the C≡N group, resulting in synergistic catalysis.
This study offers a new perspective on bifunctional catalyst design, emphasizing the role of well-defined metal pair-sites in hydrogenation reactions. The findings highlight the potential of Ni0-Niδ⁺ pair-sites in Ni₃P for efficient, cost-effective, and selective nitrile hydrogenation, opening avenues for broader applications in the fine chemical industry.