2025 AIChE Annual Meeting
(465c) Structural and Dynamical Properties of Aqueous NaCl Brines Confined in Kaolinite Nanopores
Authors
In this study, atomistic molecular dynamics simulations are applied here to study aqueous NaCl brines within 10-Å kaolinite slit pores. Kaolinite, a common clay mineral in sedimentary basins, represents an ideal model system due to its asymmetric surfaces, gibbsite, and siloxane, creating distinct nanopore chemical environments. NaCl concentrations are chosen at 5, 10, 12.5, and 15 wt.%, all below the solubility limit and high enough to provide statistically relevant information.
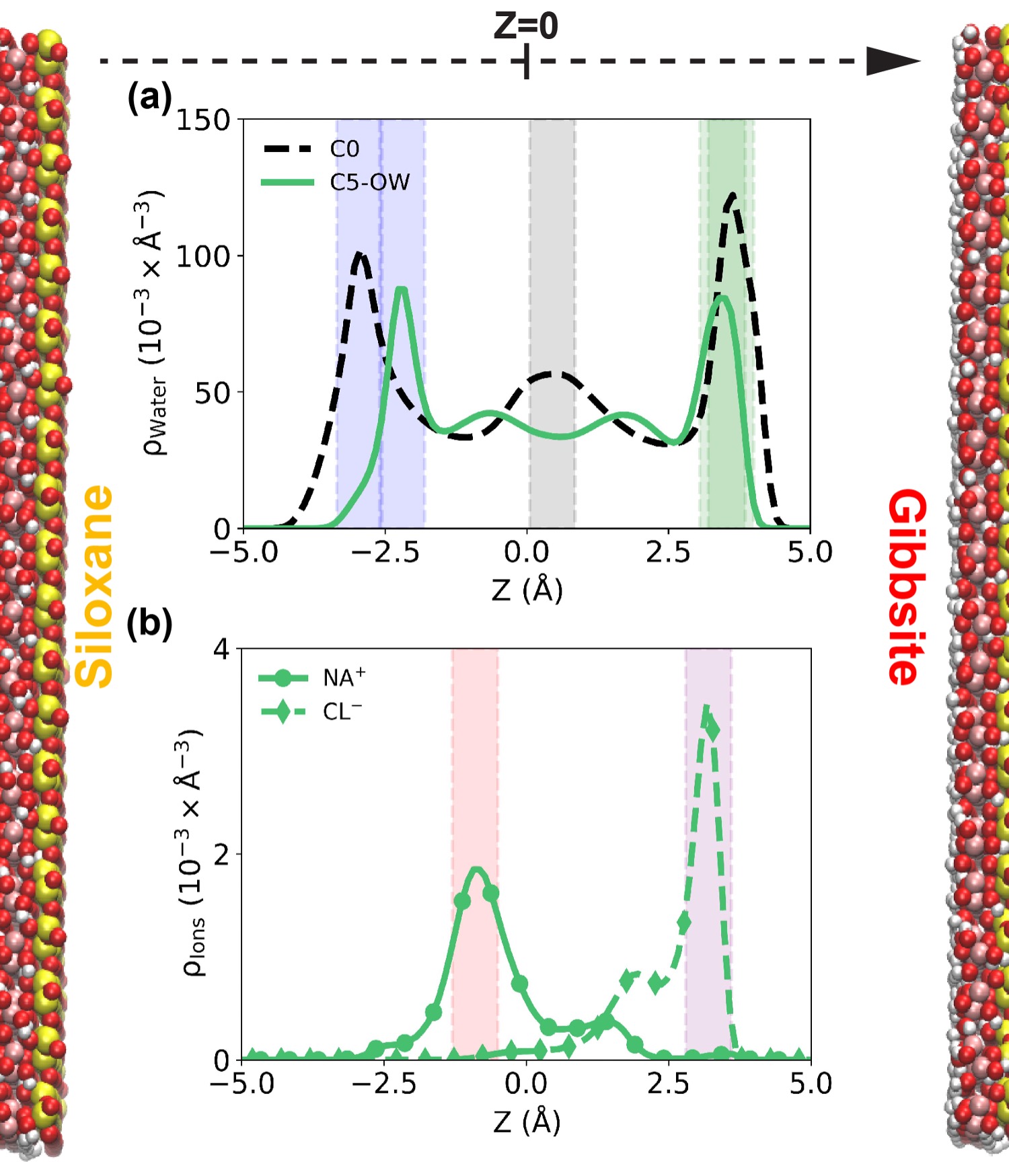
Our results reveal that the distribution of the ions within the nanopores is found not to be homogeneous. Explicitly, Na+ cations, preferentially attracted to the siloxane surface, accumulate in regions with low water density. This behavior suggests specific surface-cation interactions that override typical hydration preferences. On the other hand, Cl− anions, attracted to the gibbsite surface of kaolinite, are found within the hydration layers, indicating different interaction mechanisms for each ion type.
Confinement significantly alters the properties of both ions and water molecules compared to their bulk behavior. Explicitly, ion pairing is more pronounced within the pore than in bulk aqueous solutions at similar temperatures, pressures, and compositions. This enhanced pairing likely results from modified electrostatic screening in the confined space and the influence of surface charges. Conversely, the presence of ions affects the properties of confined water. For example, the lifetime of water–water hydrogen bonds in confinement is shortened within the hydration shells; increasing salinity from 5 to 12.5 wt.% reduces the likelihood of water density fluctuations near the kaolinite surfaces, although when the NaCl concentration rises from 12.5 to 15 wt.%, Cl− anions enhance the possibility of density fluctuations for the hydration layer near the gibbsite surface.
The simulated molecular trajectories are studied further to extract diffusion coefficients. While confinement in the kaolinite nanopore reduces the mobility of all species, non-monotonic trends are observed as a function of salt concentration. The trends seem associated with the likelihood of ion pairing. Furthermore, the diffusion coefficients for the cations are predicted to be higher than those for the anions, which is contrary to what is typically observed in bulk brines.
Because density fluctuations are strongly correlated with properties such as the solubility of gases in confined water, our observations have important implications for geo-energy applications such as UHS and CCS. These findings provide molecular-level insights that can inform macroscopic models of fluid behavior in geological formations, ultimately contributing to the design and optimization of more efficient underground hydrogen storage systems.