2025 AIChE Annual Meeting
(131c) Strategic Catalyst Design for Seawater Electrolysis: Nife Oxyhydroxide Optimization for Oer and Oxophilic Design for HER
Authors
In this work, I present two complementary studies focused on the rational design of seawater-compatible electrocatalysts. Each case highlights a data-driven computational–experimental approach to optimize either anodic or cathodic performance while preserving structural and functional stability under marine conditions.
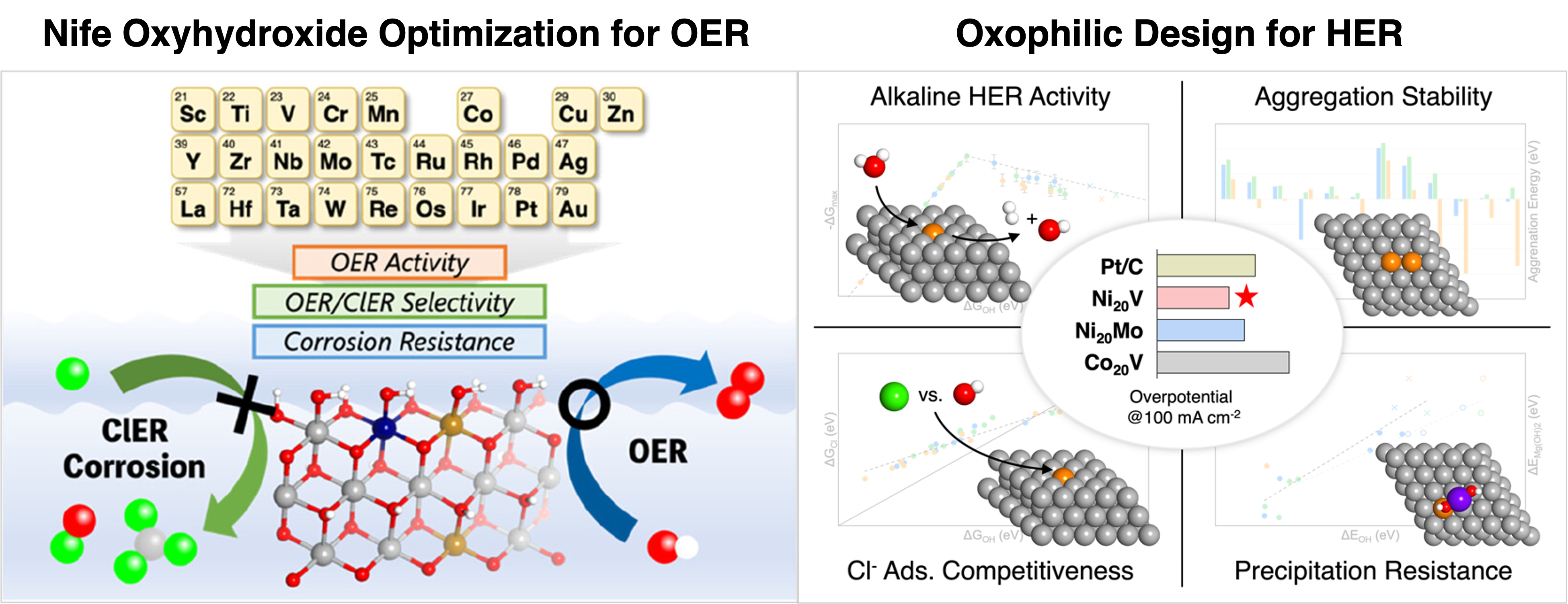
The first study targets the oxygen evolution reaction (OER) by modifying NiFe-based oxyhydroxides—one of the most active classes of non-precious OER catalysts in alkaline media. Despite their high activity, conventional NiFe oxyhydroxides suffer from severe dissolution in the presence of chloride, as Cl⁻ can oxidize metal centers and induce the formation of soluble chlorometallate species. To mitigate this, we computationally screened a set of dopants by evaluating key descriptors that capture their impact on corrosion resistance and electronic modulation. Specifically, we calculated the adsorption free energy of *OH on the doped surfaces to assess OER activity, the energy difference between metal hydroxide and metal chloride formation to evaluate Cl⁻ selectivity, and the dissolution energy of surface metal atoms to estimate structural stability. Dopants such as scandium and yttrium were identified as promising additives: they shifted the *OH binding energy closer to the optimal value (~1.6 eV), increased the energy cost of forming chlorinated species by over 0.5 eV compared to undoped NiFe, and raised the dissolution energy of Ni and Fe centers, indicating suppressed leaching under anodic conditions. These trends were corroborated by partial density of states (PDOS) analysis, which showed a shift in the Ni 3d states and reduced overlap with Cl 3p orbitals—pointing to weakened Cl adsorption.
Electrochemical experiments confirmed the benefit of these dopants. Sc-doped and Y-doped NiFe oxyhydroxide catalysts each exhibited outstanding performance under saline conditions. In 1 M KOH with 0.5 M NaCl (simulated seawater), both catalysts achieved high OER activity with an overpotential of ~270 mV at 10 mA cm⁻². Faradaic efficiencies for oxygen evolution were measured to be above 98%, and no Cl₂ evolution was detected using colorimetric tests and gas analysis. Chronopotentiometry at 10 mA cm⁻² demonstrated excellent stability over 100 hours of continuous operation. Post-mortem analysis by XPS showed suppression of Ni²⁺ and Fe³⁺ dissolution products, and STEM-EDS confirmed that the dopants remained well-dispersed in the near-surface region, helping to maintain the active phase structure. These results demonstrate that selective doping enables a substantial improvement in chlorine tolerance without sacrificing catalytic activity.
In the second study, we address the HER in alkaline seawater, where water dissociation is a critical bottleneck. Platinum, while highly active, suffers from chloride poisoning and poor scalability due to cost. Non-precious alternatives based on Ni, Co, and Cu offer a more practical path forward, but their activity is typically limited by poor water activation and surface instability. To overcome these barriers, we investigated the role of oxophilic dopants in enhancing the adsorption of hydroxyl intermediates, thereby facilitating water dissociation—the rate-limiting step in alkaline HER.
We designed a computational screening workflow that evaluated 36 host–dopant systems by integrating several key descriptors: *OH binding energy as a proxy for water activation, dopant surface stability, Cl⁻ adsorption energy to assess corrosion resistance, and compatibility with Mg²⁺/Ca²⁺ environments based on precipitation thermodynamics. Among the candidates, Ni–V and Ni–Mo systems showed an ideal balance between moderate oxophilicity, low Cl⁻ affinity, and minimal aggregation tendency.
Experimental validation confirmed the computational predictions. The Ni₂₀V catalyst achieved a remarkably low overpotential of 45 mV at 10 mA cm⁻² in 1 M KOH. At 100 mA cm⁻², it outperformed commercial Pt/C, with a smaller Tafel slope (54 mV/dec) and higher turnover frequency. In 1 M KOH with 0.5 M NaCl, Ni₂₀V maintained 97% of its initial activity after 100 hours. In 1 M KOH prepared with natural seawater from the Pacific Ocean, the catalyst sustained continuous HER operation for 200 hours with only a 2 mV increase in overpotential and negligible metal leaching as verified by ICP-OES. Electrochemical impedance spectroscopy (EIS) indicated improved charge transfer kinetics compared to undoped Ni. Spectroscopic and microscopic analyses confirmed that V remained well-dispersed throughout the testing. SEM images taken before and after operation revealed no significant morphological changes, and no visible Mg(OH)₂ or CaCO₃ precipitates were observed on the catalyst surface.
These two studies demonstrate a unified approach to seawater electrolysis: leveraging electronic structure modulation, adsorption energetics, and corrosion analysis to engineer catalysts for both OER and HER. While the anode design focuses on suppressing ClER through dopant-induced oxidation potential shifts, the cathode strategy emphasizes the role of surface oxophilicity in promoting water activation and maintaining long-term resistance to seawater impurities.
Importantly, both efforts go beyond conventional activity benchmarking. Our catalyst candidates were evaluated not only in idealized alkaline electrolytes, but also under conditions that mimic or replicate realistic seawater operation. Performance metrics included not just current–voltage behavior, but also Faradaic efficiency, long-term durability, and chemical stability under ionic interference. These stringent criteria reveal the importance of designing materials with multiple performance dimensions in mind, especially for systems destined for real-world implementation.
Relative to prior literature, our work introduces a comprehensive, mechanistically informed catalyst design workflow that is transferable across different electrochemical reactions. For OER, while previous studies have explored NiFe-based systems, they have rarely incorporated selective dopants that simultaneously preserve activity and inhibit Cl⁻ oxidation under realistic saline conditions. For HER, while some dopant strategies have been reported, few have systematically evaluated Cl⁻ and divalent cation tolerance alongside activity and stability in true seawater. By including these practical considerations, our design principles are more aligned with the operational challenges faced in actual seawater electrolysis.
Taken together, these studies offer a pathway to low-cost, precious-metal-free catalysts with strong activity and resilience in complex environments. The methodologies and insights developed here can be applied to broader applications such as brine electrolysis, industrial wastewater splitting, or electrolyzers integrated into coastal energy systems.
This work reflects a broader philosophy of catalysis research that combines theoretical insight, data-driven screening, and rigorous experimental testing under realistic operating conditions. By connecting fundamental electronic descriptors to macroscopic performance and degradation behavior, this research advances the foundation for building the next generation of durable and scalable electrochemical systems.