2025 AIChE Annual Meeting
(72d) Spontaneous Supramolecular Assembly of Charge-Paired Nanocarriers Constructed from Lipids and Proteins for Efficient and Non-Toxic siRNA Transfection
Authors
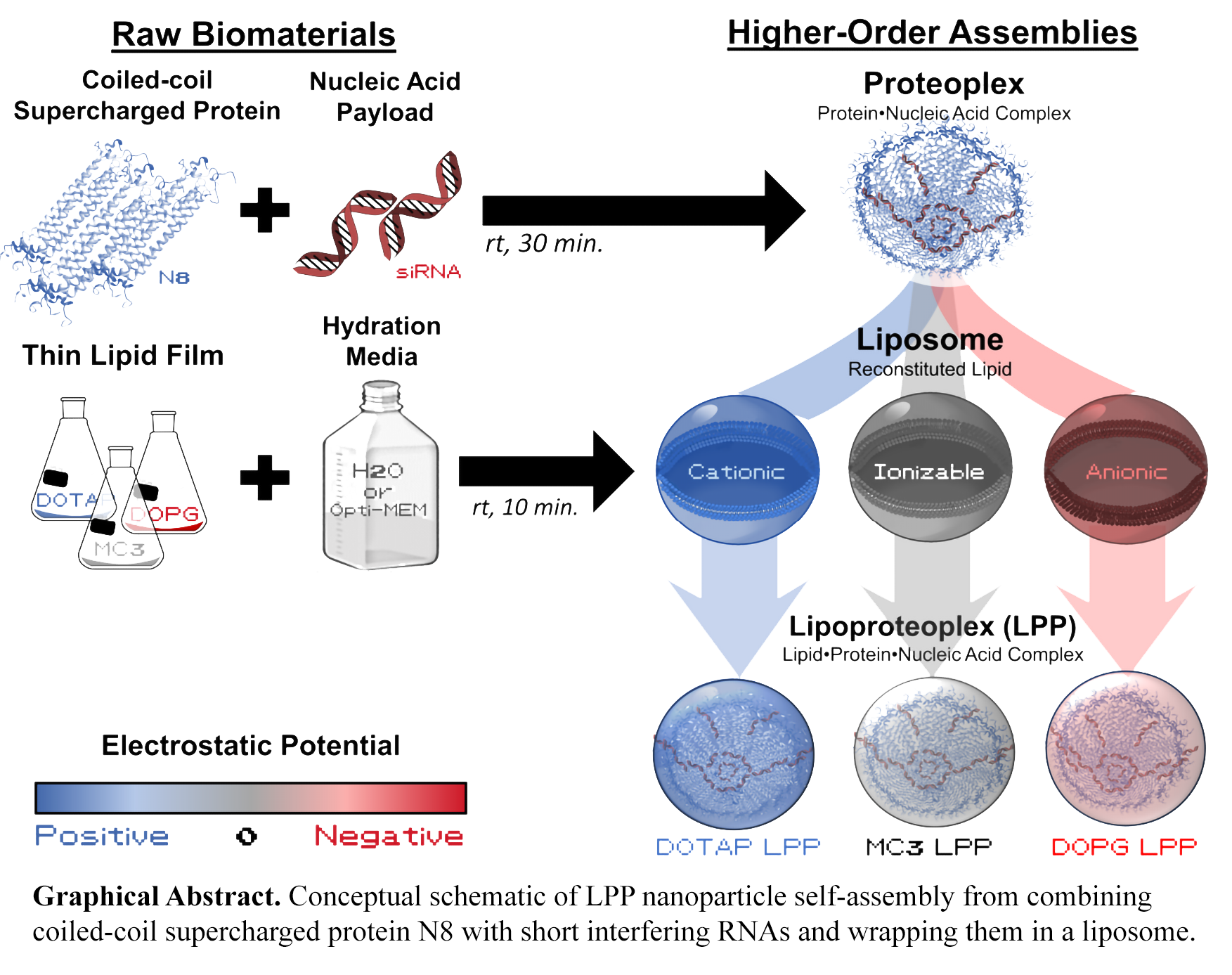
In this study, we investigated the supramolecular self-assembly of ternary lipoproteoplex (LPP) nanoparticles formulated from combining (i) lipids, (ii) positively-charged N8, and (iii) negatively-charged short interfering RNA (siRNAs). DOTAP, Dlin-MC3-DMA, and DOPG were selected for our lipid comparison study due to their widespread available, well-documented properties, and structural similarities as two-tailed lipids with comparable molecular weight and degrees of unsaturation. Critically, these lipids differ by head group charge—DOTAP is cationic, Dlin-MC3-DMA is ionizable, and DOPG is anionic—allowing for a systematic investigation of how lipid charge influences nanoparticle assembly and function. While most commercial formulations incorporate complex blends of multiple lipids, sterols, PEGylated agents, and other excipients, our study deliberately focuses on simplified formulations to better understand the fundamental phenomenological interactions between the charged wrapper, protein-based excipients, and the nucleic acid payload.
Reconstituting dried lipid films of DOTAP, Dlin-MC3-DMA, or DOPG with solutions of protein-bound siRNA in a 4:3:1 ratio resulted in discrete nanoparticles of spherical morphology, ranging 150-250 nm in diameter as determined through a combination of dynamic light scattering and transmission electron microscopy. Increasing the excess of either protein or lipid did not alter the resulting nanoparticle size, suggesting that the system reached a quasi-equilibrium assembly state within 30 minutes of incubation. Using a size-exclusion method to isolate nanoparticle assemblies from unincorporated components, we quantified the amount of N8 and siRNA in the permeate using commercial Quantifluor® RNA System and Pierce® Bicinchoninic Acid Assay kits. Depending on the degree of charge complementarity between components, incorporating N8 into LPP nanoparticles appeared to increase siRNA loading efficiency by approximately 4- to 10- fold. Interestingly, zeta potential measurements showed the final surface charge of the LPP complexes reflected the charge of the payload, not just the lipid, suggesting that residual N8 or siRNA adsorbs onto the nanoparticle surface to form a corona. Through a time-course transfection assay performed in NIH-3T3 cells stably expressing a fluorescent reporter, we observed 2- to 7-fold higher gene knockdown in vitro when using LPPs compared to liposomes without the N8 protein excipient. Minimal differences in cytotoxicity following the 48-hour treatment period were observed between formulations made with the same lipid but with or without N8, indicating that N8 does not add significant toxicity to the overall safety profile of the particle. Ultimately, this suggests that lipid surface charge plays a more dominant factor in determining cell compatibility. Collectively, these findings provide valuable insights into the electrostatic interactions governing LPP self-assembly and offer guidance for formulating ternary delivery systems with charged, non-viral biomaterials.