2025 AIChE Annual Meeting
(252f) Spectroscopic Insights into Ni Catalysts for CO2 Methanation
Authors
Madelyn R. Ball - Presenter, West Virginia University
Majed Alam Abir, West Virginia University
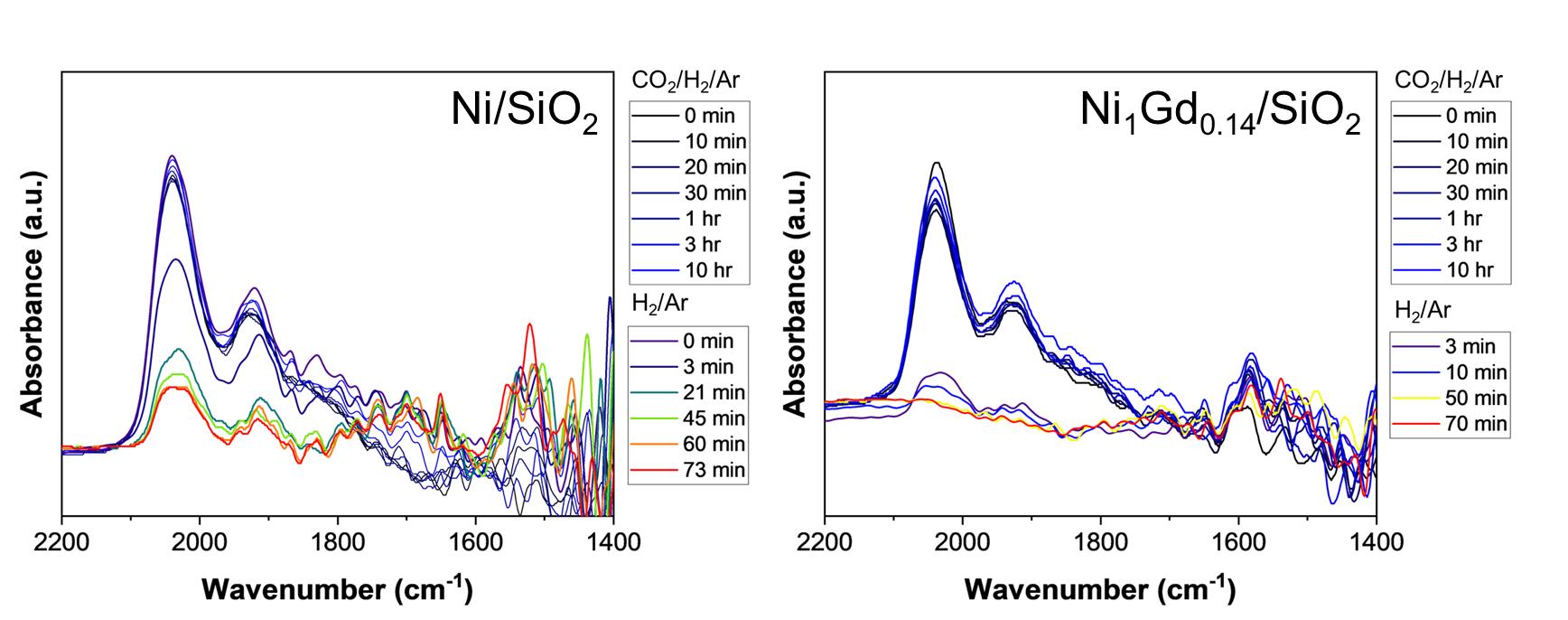
Ni catalysts have been demonstrated to be active and selective for CO2 hydrogenation to methane. As CO production via the reverse water gas shift reaction is favored at higher temperatures, we have investigated routes for increasing catalytic activity at low temperature to maintain high CH4 selectivity. We have investigated the role of a Gd promoter over a set of catalysts with Gd:Ni molar ratios between 0.07 and 1.3. We determine that for catalysts with the same Ni loading, site densities do not change with increasing Gd loading. We observe a 3x increase in turnover frequency for Ni1Gd0.14/SiO2 compared to monometallic Ni/SiO2. This increased activity is due, in part, to an increase in basic sites which facilitate adsorption and dissociation of CO2. Via in situ infrared spectroscopy, we observe carbonyl species present on all catalysts, and formate species present on all Gd containing catalysts under reaction conditions. This observation suggests that Gd modulates the favored reaction pathway for methane production. To further evaluate whether these carbonyl and formate species are spectator or reactive intermediates, we removed CO2 from the gas feed and observe a decrease in peak intensities over ~1 hour. In the case of monometallic Ni, carbonyl species remain on the catalyst surface, while for the NiGd catalysts, no carbonyl species are present after 1 hour without CO2 in the feed. These experiments indicate the presence of strongly adsorbed and non-reactive CO species on the surface of the monometallic Ni catalyst, while in the presence of Gd these species are not observed. These strongly adsorbed CO species lead to the lower observed activity on Ni/SiO2, as well as lower stability compared to catalysts with Gd.