2025 AIChE Annual Meeting
(517a) Spatially Coupled Reactions in Oxidative Bi-Reforming: Impact of Feed Composition and Temperature on CH4 and CO2 Conversion on Pt/Pd/Al2O3 Monolith
Authors
OBR combines four key reactions in a single reactor:
R1: (-802 kJ/mol CH4) – Total Oxidation of Methane
R2: (+206 kJ/mol CH4) – Steam Methane Reforming
R3: (+247 kJ/mol CH4) – Dry Methane Reforming
R4: (-41.1 kJ/mol) – Water Gas Shift Reaction
The coupled approach enables the conversion of CH4, and CO2 simultaneously as compared to conventional steam or dry reforming [7-11]. H2O and O2 are added to the system to mitigate coke formation [7-12]. O2 can react with CH4 directly, increase the temperature and thus allows more CH4 and CO2 conversion by reforming but at the same it will also produce CO2 by total oxidation.
The experimental reactor setup comprises a feed system that delivers a gas mixture containing CH4, O2, CO2, H2O, and inert (N2 or Ar). A mass spectrometer and FTIR provide effluent composition data over a range of feed compositions, temperatures, and flow rates. Two spatially resolved measurement methods are utilized. Spatial temperature is measured using Optical Frequency Domain Reflectometry (OFDR) [5,6] while reacting species concentrations are measured by capillary inlet mass spectrometry (SpaciMS) [3,4,5]. This data provides a detailed assessment of the catalyst performance over a range of application-relevant conditions.
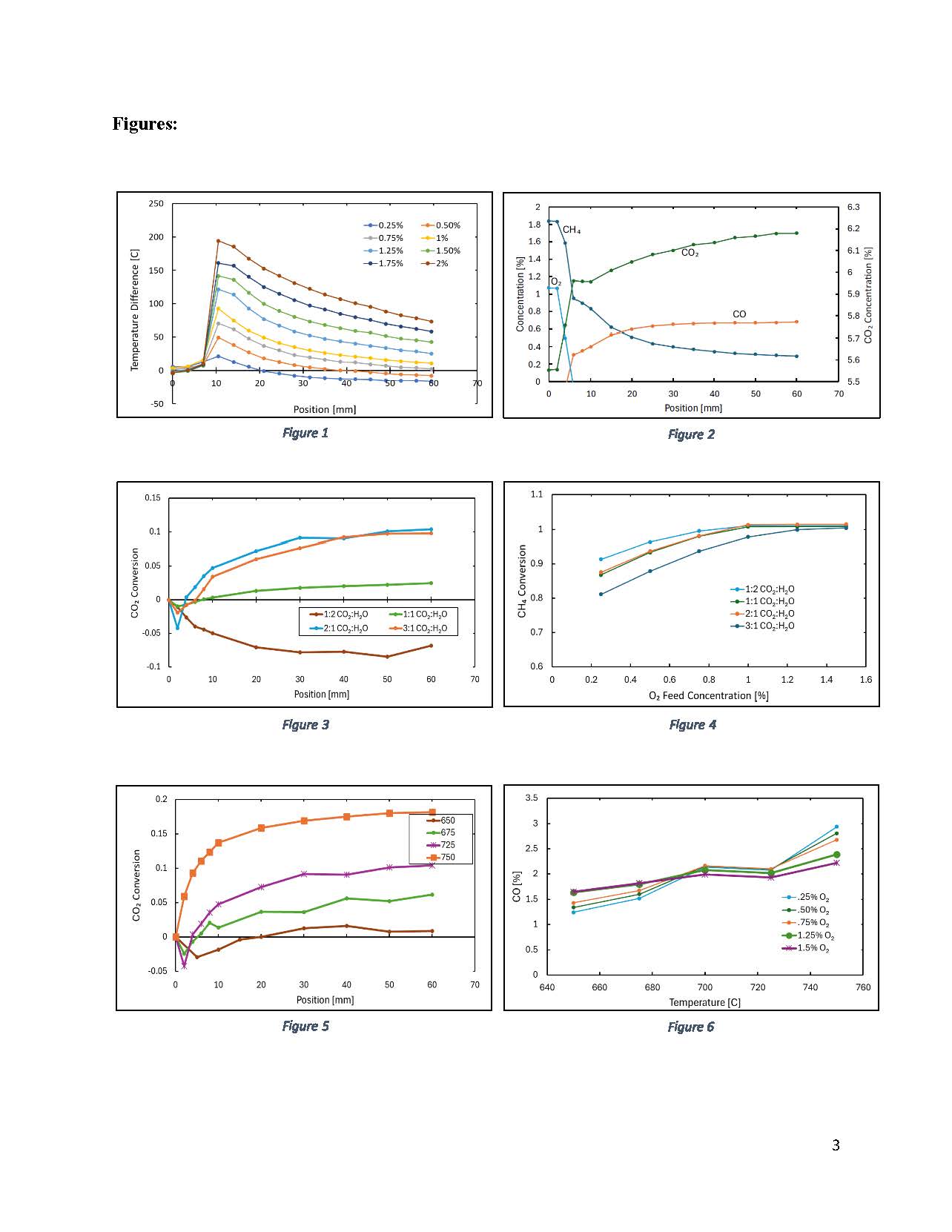
Spatial analysis of OBR shows a strong coupling between exothermic methane oxidation and the endothermic reforming / reverse WGS reactions. The temperature profiles are clearly divided into two zones: (1) total oxidation zone and (2) endothermic reaction zone. Fig. 1 shows spatial temperature profiles along the catalyst for different oxygen concentrations ranging 0.5 – 2.0%. Fig. 2 shows the concentration profile that corresponds to temperature profile shown in Fig. 1. The data reveals that a hotspot forms at the front face of the monolith and that the methane oxidation zone is confined to the first ~10 mm of monolith. Beyond that point, there is a gradual decrease in temperature due to slow endothermic reactions.
CO2 is converted from reverse WGS instead of dry reforming of CH4. This conclusion can be further strengthened from the fact that higher feed water concentrations negatively impacted CO2 conversion. Further experiments are in progress to strengthen this conclusion at higher CH4 and CO2 concentrations.
Fig. 3 shows an interesting spatial trend of CO2 conversion for different CO2 / H2O. The data reveal that conversion of CO2 strongly depends on this ratio because as the water content is increased, it shifts the equilibrium towards the forward WGS reaction.
Fig. 4 shows CH4 conversion with varying oxygen content as well as CO2/ H2O. Two trends are noted: (1) For each CO2 / H2O ratio, there is an increase in CH4 conversion which is due to increased oxidation as well as steam reforming. (2) For lower CO2/ H2O ratio, CH4 conversion is higher due to a higher SMR rate resulting from a higher water content. This trend is more prominent in lower oxygen content.
Fig. 5 shows the temperature effect on the spatial CO2 conversion profile. The data reveals that there is CO2 production at the front face which shows total oxidation of methane which is also evident from the spatial temperature profiles. The conversion of CO2 is increased by the increase in temperature due to reverse WGS which is the dominant reaction pathway; this is evident from different CO2 to H2O ratio effects.
Fig. 6 shows the temperature effect on CO concentration with varying feed O2 concentration. Two trends are noted: (1) For a fixed O2 concentration, there is an increase in CO concentration which is due to an increased rate of reforming reactions. The high temperature favors reverse WGS which also contributes to CO production. (2) At 650°C, CO production is increased by increasing the O2 content; as CH4 is not fully converted, there is still a potential for reforming reaction. When O2 is increased, total oxidation increases the system temperature which promotes reforming reaction and more CO production. This trend inverts at higher temperatures like at 750°C where CH4 is already fully converted. In this case when O2 is increased, it increases the total oxidation but there is not enough methane for reforming reaction so total oxidation just reduces the share of reforming reaction producing less CO.
One of the significant challenges encountered at high temperatures and elevated reactant concentrations is the increased tendency for coke formation. This may lead to catalyst deactivation, ultimately affecting the efficiency of the overall reaction process. Experiments are currently underway that quantify the extent of coke formation and measures to mitigate its effects.
References:
- IEA, "Methane and climate change," (n.d.). https://www.iea.org/reports/methane-tracker 2021/methane-and-climate-change (accessed June 18, 2024).
- Song, W. Pan, Tri-reforming of methane: A novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios, in: Catal Today, 2004. https://doi.org/10.1016/j.cattod.2004.09.054.
- Nguyen, M.P. Harold, D. Luss, "Spatiotemporal behavior of Pt/Rh/CeO2/BaO catalyst during lean-rich cycling," Chemical Engineering Journal 262 (2015) 464–477. https://doi.org/10.1016/j.cej.2014.09.103.
- Nguyen, P.Y. Peng, D. Luss, M.P. Harold, "Assessing intrusion by the capillary during spatially resolved mass spectrometry measurement," Chemical Engineering Journal 307 (2017) 845–859. https://doi.org/10.1016/j.cej.2016.08.101.
- Y. Peng, H. Nguyen, M.P. Harold, D. Luss, Spatio-Temporal Phenomena in Monolithic Reactors Measured by Combined Spatially-Resolved
- Mass Spectrometry and Optical Frequency Domain Reflectometry, in: Advances in Chemical Engineering, 2017. https://doi.org/10.1016/bs.ache.2017.07.001.
- Nguyen, M.P. Harold, D. Luss, "Optical frequency domain reflectometry measurements of spatio-temporal temperature inside catalytic reactors: Applied to study wrong-way behavior," Chemical Engineering Journal 234 (2013) 312–317. https://doi.org/10.1016/j.cej.2013.08.074.
- Pham Minh, X.H. Pham, T.J. Siang, D.V. N. Vo, "Review on the catalytic tri-reforming of methane - Part I: Impact of operating conditions, catalyst deactivation and regeneration," Appl Catal A Gen 621 (2021) 118202. https://doi.org/10.1016/j.apcata.2021.118202.
- Soleimani, M. Lehner, "Tri-Reforming of Methane: Thermodynamics, Operating Conditions, Reactor Technology and Efficiency Evaluation—A Review," Energies (Basel) 15 (2022). https://doi.org/10.3390/en15197159.
- Rezaei, L.J.J. Catalan, "Evaluation of CO2utilization for methanol production via tri-reforming of methane," Journal of CO2 Utilization 42 (2020). https://doi.org/10.1016/j.jcou.2020.101272.
- B.H. Nguyen, E. Zondervan, "Methanol production from captured CO2 using hydrogenation and reforming technologies- environmental and economic evaluation," Journal of CO2 Utilization 34 (2019) 1–11. https://doi.org/10.1016/j.jcou.2019.05.033.
- Halmann, A. Steinfeld, "Fuel saving, carbon dioxide emission avoidance, and syngas production by tri-reforming of flue gases from coal- and gas-fired power stations, and by the carbothermic reduction of iron oxide," Energy 31 (2006) 3171–3185. https://doi.org/10.1016/j.energy.2006.03.009.
- D. Alli, P.A.L. de Souza, M. Mohamedali, L.D. Virla, N. Mahinpey, "Tri-reforming of methane for syngas production using Ni catalysts: Current status and future outlook," Catal Today 407 (2023) 107–124. https://doi.org/10.1016/j.cattod.2022.02.006.