2025 AIChE Annual Meeting
(180ar) In-Situ Raman Spectroscopy of Water Phase Behavior during Interaction with Silica (SiO?) and Saline Solutions
Authors
Amol Pophali - Presenter, Stony Brook University
Krishnakumari Pamula, Stony Brook University
Dilip Gersappe, Stony Brook University
Tae Jin Kim, Stony Brook University
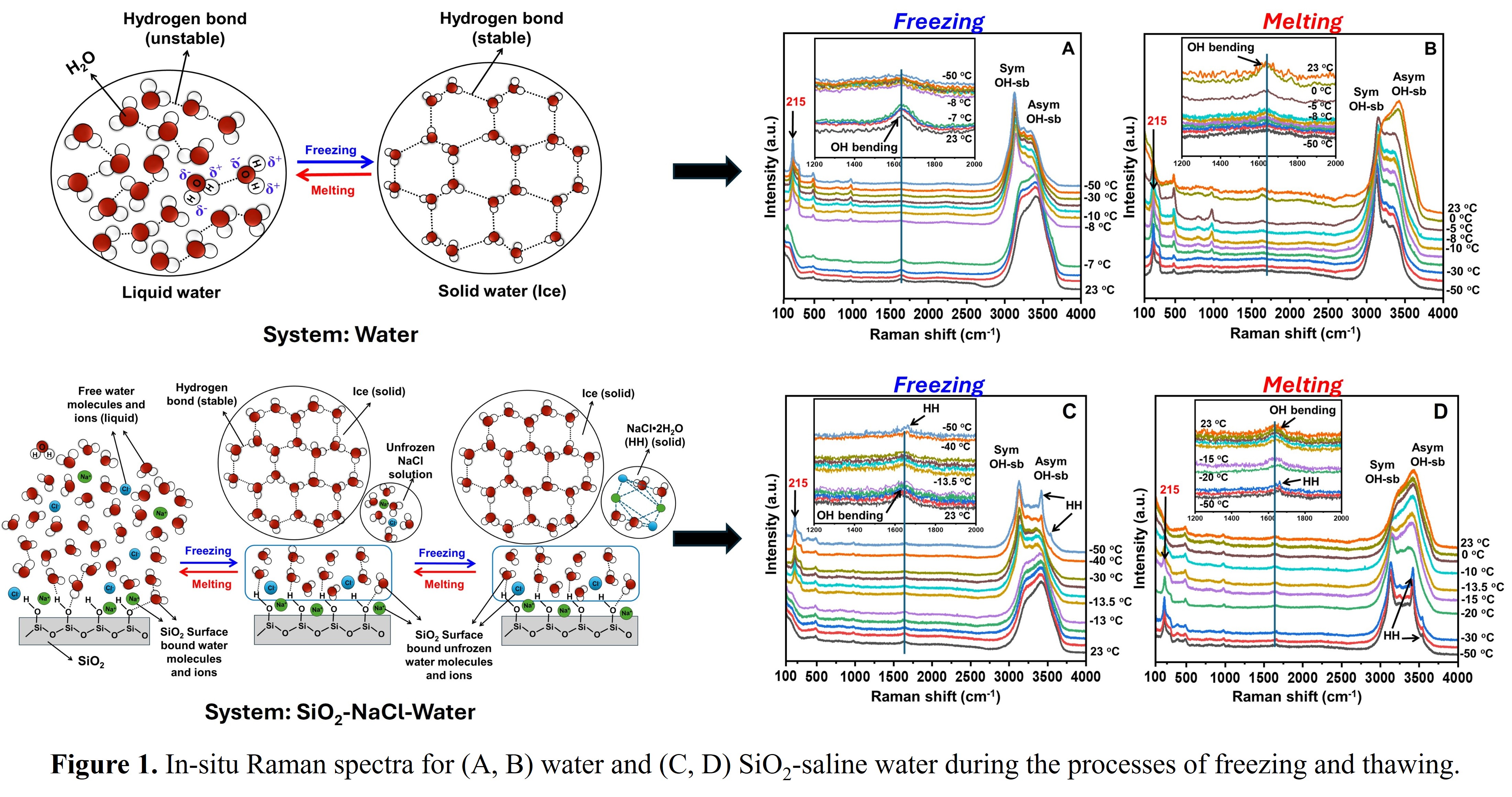
With climate change and its associated fluctuations, approximately 35% of Earth's land surface has been severely affected [1]. To address these issues in the future, it is crucial to study a fundamental understanding of molecular level structural changes in soil during temperature fluctuations. Silicon dioxide (SiO2), a major geological component of soil, along with water (H2O) and salinity (NaCl), were considered as influencing factors. Phase transition temperatures of pure water and aqueous NaCl solutions, both in bulk form and mixed with SiO2 powder, were investigated using in-situ Raman spectroscopy. The freezing and melting temperatures were determined by analyzing the OH-stretching and bending regions of the Raman spectrum, alongside investigating the formation of hydrohalite (HH) in saline water. A spectral phase transition marker, SD, defined as the intensity ratio of asymmetric to symmetric OH-stretching bands (Iasym/Isym), was applied to measure the freezing and melting temperatures. In the case of bulk water (Fig. 1A and B) and aqueous salt solutions (not shown for brevity), complete transformation of liquid to solid phase was observed. In the case of SiO2 mixed liquids, however, a non-freezable liquid layer was observed, which could be due to the interaction between the silanol (Si-OH) functional groups and the water/NaCl solution (Fig. 1C and D). These findings are expected to provide valuable insights into the freezing and thawing processes in both normal and saline soil in cold regions.
Reference
- Qi, J., Zhang, X. & Wang, Q. Hydrol. 571, 605–618 (2019).