2025 AIChE Annual Meeting
(382af) In-Situ Raman and 2D-COS Analysis of Molybdenum Deposition on TiO2 Via Equilibrium Deposition Filtration
Authors
Understanding precursor-support interactions during catalyst preparation is crucial for optimizing metal dispersion and surface reactivity. In this study, we employed equilibrium deposition filtration (EDF) to investigate the pH-dependent adsorption of molybdenum species onto commercial TiO₂ pellets. Real-time, in-situ Raman spectroscopy was used to monitor molybdenum speciation during deposition, while two-dimensional correlation spectroscopy (2D-COS) was applied to enhance spectral interpretation and reveal mechanistic insights into the deposition process.
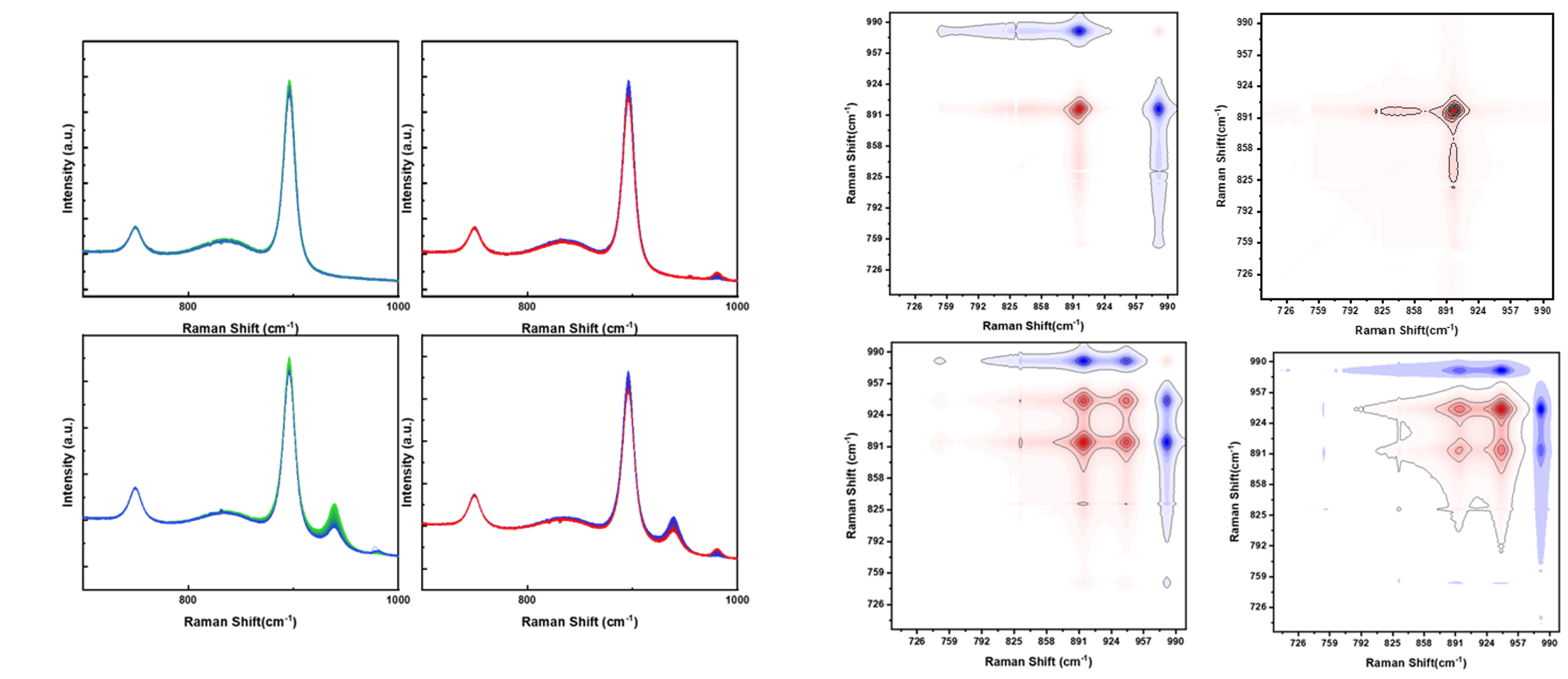
At different pH values, distinct molybdenum species were observed: monomers (MoO₄²⁻) at 896 cm⁻¹, oligomers (Mo₇O₂₄⁶⁻) at 939 cm⁻¹, and polymers at 969 cm⁻¹. The leaching of sulfate from untreated TiO₂ was tracked via the 980 cm⁻¹ Raman band. While traditional spectral analysis provides only qualitative trends, 2D-COS enables a more precise deconvolution of overlapping bands and reveals the temporal sequence of spectral changes under perturbation—in this case, time.
Synchronous 2D-COS spectra show correlation peaks indicating co-varying intensities among different molybdenum species and sulfate groups, while asynchronous spectra provide insight into the order of events. For example, the cross-peaks between 896 and 980 cm⁻¹ demonstrate that sulfate leaching precedes monomer adsorption. At lower pH, the emergence of strong asynchronous peaks between 896 and 939 cm⁻¹ indicates the transformation and sequential uptake of monomers and oligomers. Auto-power spectra also quantify the intensity variation of individual species throughout the deposition. Time-resolved 2D-COS maps segmented into 30-minute intervals further reveal that the majority of adsorption occurs within the first 2.5 hours of deposition and reaches equilibrium thereafter.
Kinetic modeling of Raman-derived concentrations using pseudo-first-order, pseudo-second-order, and Elovich models confirms that the pseudo-second-order model best describes the adsorption process, consistent with chemisorption on a heterogeneous surface.
This work demonstrates the power of coupling EDF with in-situ Raman and 2D-COS to uncover molecular-level deposition dynamics. The approach offers valuable guidance for tailoring catalyst supports and deposition conditions for improved catalyst design.