2025 AIChE Annual Meeting
(37d) In-Situ Monitoring and Solubility Modeling of Sodium Phosphate Using Online PAT Tools
Authors
In this study, Process Analytical Technology (PAT) tools were employed to monitor the forms of sodium phosphate that crystallize from cooling crystallization at different concentrations, temperatures, and cooling rates. Focused Beam Reflectance Measurement (FBRM) was utilized to approximate the crystal growth rate and overall chord-length distribution in real-time and a Mettler Toledo EasyViewer probe visually inspected the crystals, differentiating observed morphologies. Raman Spectroscopy was used to analyze the solid and solution phases; Raman spectroscopy can distinguish phosphate (PO43-) and hydrogen phosphate anions (HPO42-) in the solution phase while also detecting solid crystals. Attenuated Total Reflectance – Fourier Transform Infrared (ATR-FTIR) spectroscopy measured the solution phase only; ATR-FTIR absorbance varies directly with solute concentration, offering insights into dissolved phosphate ions.
Identification of sodium phosphate crystalline phases formed under specific conditions was facilitated through characterization using X-ray Diffraction (XRD), Differential Scanning Calorimetry/Thermogravimetric Analysis (DSC/TGA), and Raman spectroscopy. XRD analysis suggests that the crystallized phases may include disodium hydrogen phosphate or hydrated trisodium phosphate. Interestingly, although in-situ spectroscopic data predominantly show PO43- in solution, XRD analysis of filtered and dried solids indicates that partial protonation occurred during or after filtration or drying, yielding HPO42- phases that are not directly evident from the solution phase measurements. Meanwhile, PAT tools effectively tracked sodium phosphate phase transitions in real-time, with Raman and FTIR spectroscopy enabling the distinction between PO43- and HPO42- during hydrolysis.
The solubility model closely reproduces the experimental solubility trends across the tested temperature range, indicating that the fitted Pitzer parameters capture the non-ideal behavior of the system. Furthermore, the application of temperature-dependent expressions for β(0), β(1) and C(φ), reveals that ion-ion and ion-solvent interactions in the Na3PO4-NaOH-H2O exhibit a nonlinear dependence on temperature. While the model fits the current system well, it is parameterized for a fixed NaOH concentration (3 mol/kg) and assumed the crystallization phase is Na3PO4·xH2O. Extrapolation to significantly different concentrations or solid phases would require reparameterization.
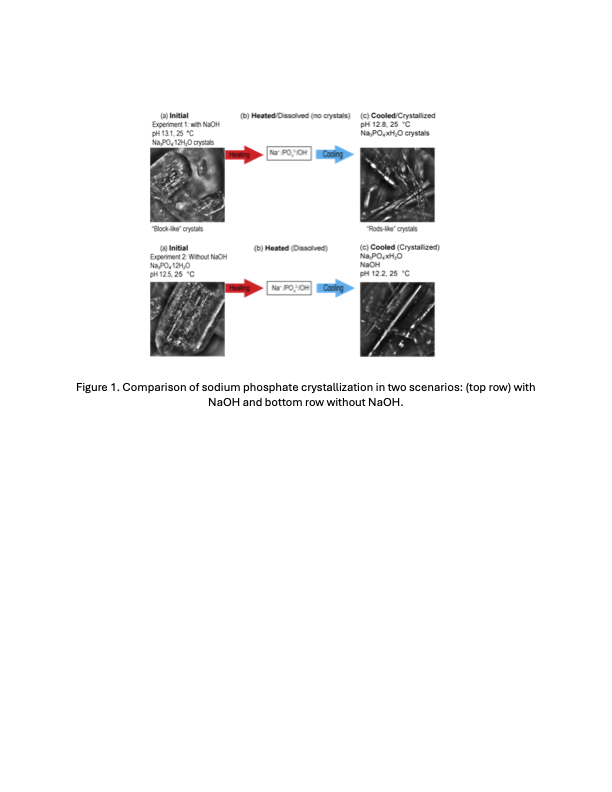
Ongoing work focuses on experiments conducted under two scenarios: (1) unadjusted pH, where no acid or base is added beyond the initial conditions, and (2) NaOH added experiments, where NaOH is introduced at 25 °C. Preliminary results indicate that whether NaOH is added during heating (25 -55 °C) or not, PO43_ remains the predominant species. Upon cooling, the final crystals exhibit the same morphology in both cases (see Figure 1). In the context of Hanford’s waste processing, this knowledge can help to understand phosphate solubility and potentially mitigate the costs and delays caused by phosphate crystallization in pipelines
[1] D. B. Tren R. Graham, Emily T. Nienhuis, Winnie Liu, Xiadong Zhao, Mark E. Bowden, Ashley R. Kennedy, Teresa L. Lemmon, Marie S. Swita, Jacob I. Morton, Hsiu-Wen Wang, Michael Britton, and Carolyn I. Pearce, "Determining solubility of aluminum and phosphate gels in Hanford Waste," presented at the WM2025 Conference, Phoenix, Arizona, March 9-13, 202, 25315.