2025 AIChE Annual Meeting
(309b) In-Situ Activation of Pre-Catalyst Improves CO2 Conversion to Ethanol in Pulsed Electrolysis
Authors
Meenesh Singh, University of Illinois At Chicago
Haitham Bery, Assiut University
Ayush Karwa, University of Illinois
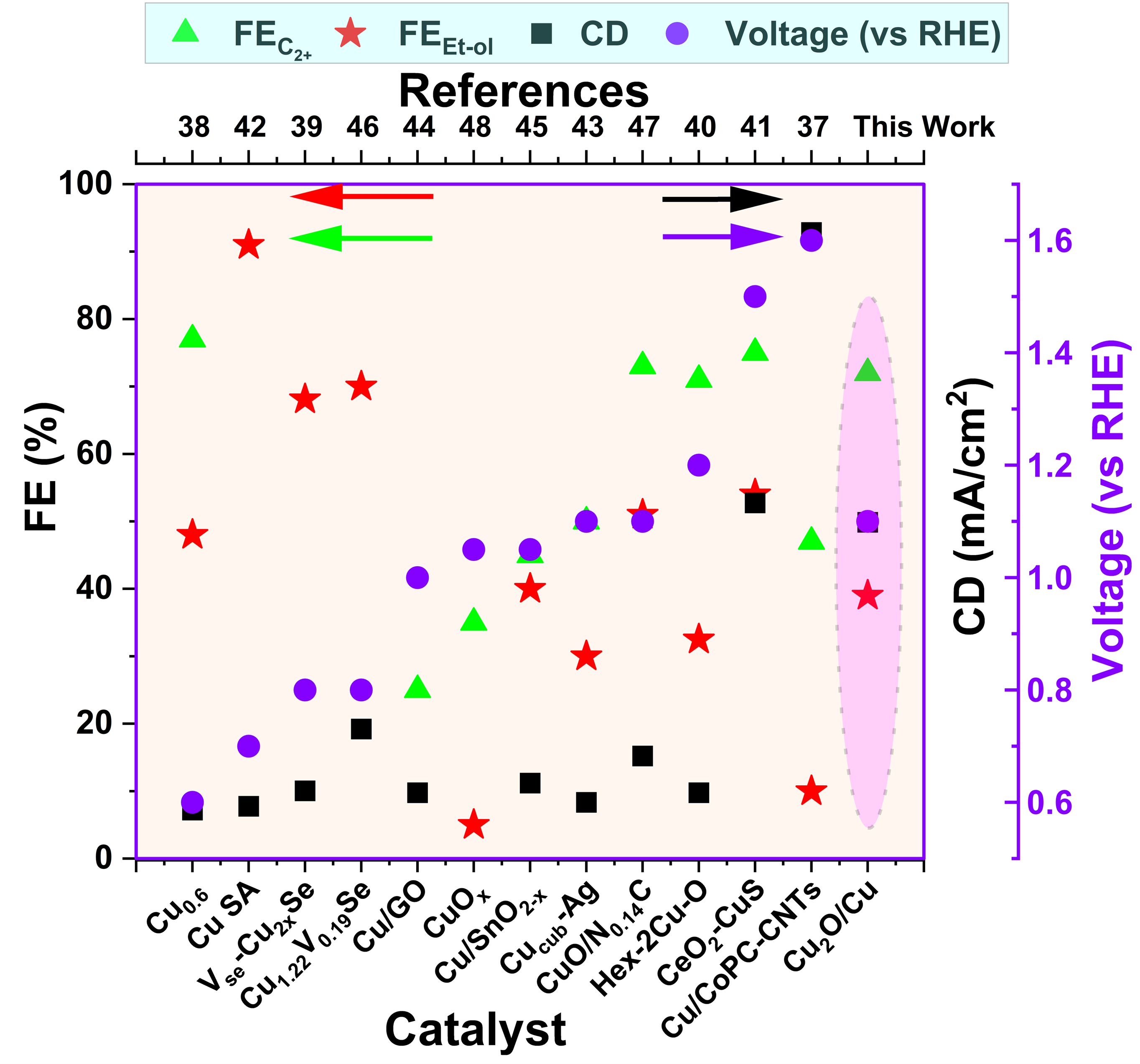
Electrochemical conversion of dissolved CO2 in bicarbonate electrolytes, i.e., bicarbonate electrolysis, offers distinct advantages over gas diffusion electrode systems by allowing direct utilization of CO2 capture solution as electrolyte while bypassing the energy-intensive CO2 release and by eliminating the need for CO2 separation and purification. However, bicarbonate electrolysis is prone to CO2 mass transfer limitations and local pH-driven CO2 depletion. Pulsed electrolysis with resting time > 10 sec has increased CO2 concentration, but the required cathodic potentials remained very high (e.g., -1.6 V vs RHE for 300 mA/cm2). The higher potential often causes catalyst surface reorganization, gradually losing active sites and variations in selectivity to CO2 reduction reaction (CO2RR). Here, we report directed pre-catalyst evolution via an in-situ activation method that allows pre-catalysts to equilibrate under dynamic (pulsed) electrolysis conditions before employing it for CO2RR. We demonstrate in-situ activation of Cu2O/Cu mesh, which under short-width (t = 4s) pulsed electrolysis provides stable mixed oxidation states of Cu, favoring ethanol-rich crude mixture formation. The pulse electrolysis waveform consisting of six distinct segments is tuned to form Cu1+ oxides, followed by its reduction to generate local alkaline conditions favoring C-C coupling. This synergistic effect results in FEs of 72% for C2+ products, 52% for total liquid products, and 39% for ethanol at an applied current density of 150 mA/cm2 and a cathodic potential of -1.1V vs. RHE. The in-situ cyclic voltammetry confirms the formation of Cu2+/Cu1+, whereas ex-situ elemental and structural analysis show a near-stable state of post-electrolysis samples. The 1D electrochemical model explains the role of pulsed CO2RR in dynamic local pH and enhanced CO2 concentration gradients near the catalyst/electrolyte interface, which helps steer selectivity towards C2+ products. Overall, this study provides new insights into the synergistic effect of in-situ activation of pre-catalyst and pulsed electrolysis for higher selectivity towards C2+ products.