2025 AIChE Annual Meeting
(214d) Simultaneous Productions of Ethylene and Ammonia Using a Microwave-Thermal Heating Hybrid Reactor
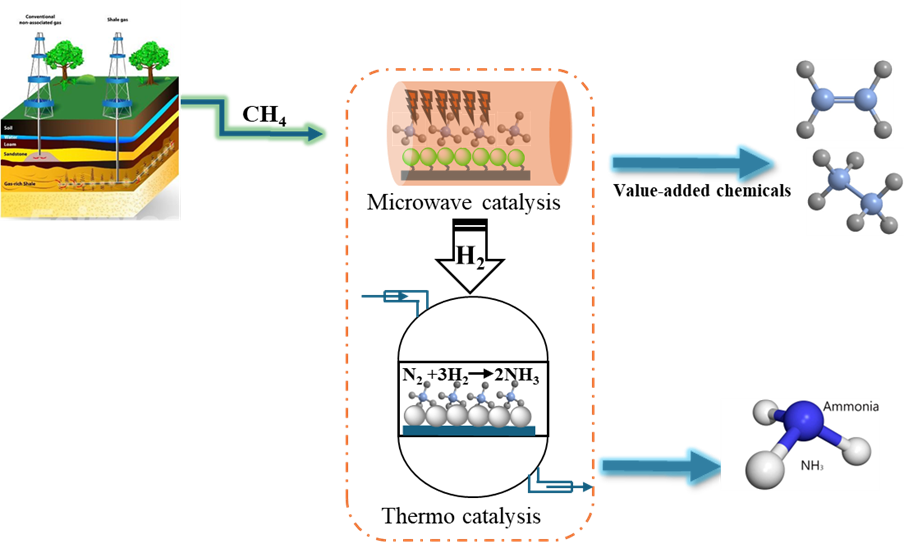
The hybrid reactor is designed to address the temperature mismatch between the two reactions. Methane coupling requires high temperatures (above 700°C), whereas ammonia synthesis typically occurs at lower temperatures (around 350°C). To overcome this challenge, we utilize microwave heating for methane coupling with a CsMo/CeO₂ catalyst and thermal heating for ammonia synthesis with a CsRu/CeO₂ catalyst. The methane activation process produces ethylene as well as hydrogen (H₂) through CH₄ decomposition. The generated H₂ is then used in the exothermic NH₃ synthesis reaction, effectively utilizing the methane not only for ethylene production but also for providing the necessary hydrogen for ammonia synthesis.
Various characterization techniques were employed to understand catalyst behavior and reaction mechanisms. In-situ Raman spectroscopy provided real-time insights into catalyst dynamics, while X-ray diffraction (XRD) and transmission electron microscopy (TEM) were used to analyze the structural and morphological properties. Hydrogen temperature-programmed reduction (H₂-TPR) experiments were conducted to assess catalyst reducibility and active site distribution.
This hybrid reactor configuration offers a promising route for the simultaneous production of ethylene and ammonia, optimizing methane utilization in chemical manufacturing and enabling process intensification