2025 AIChE Annual Meeting
(185p) Side Chain Engineering of Anion-Exchange Ionomers Enables Selective CO2 Electrolysis to CO
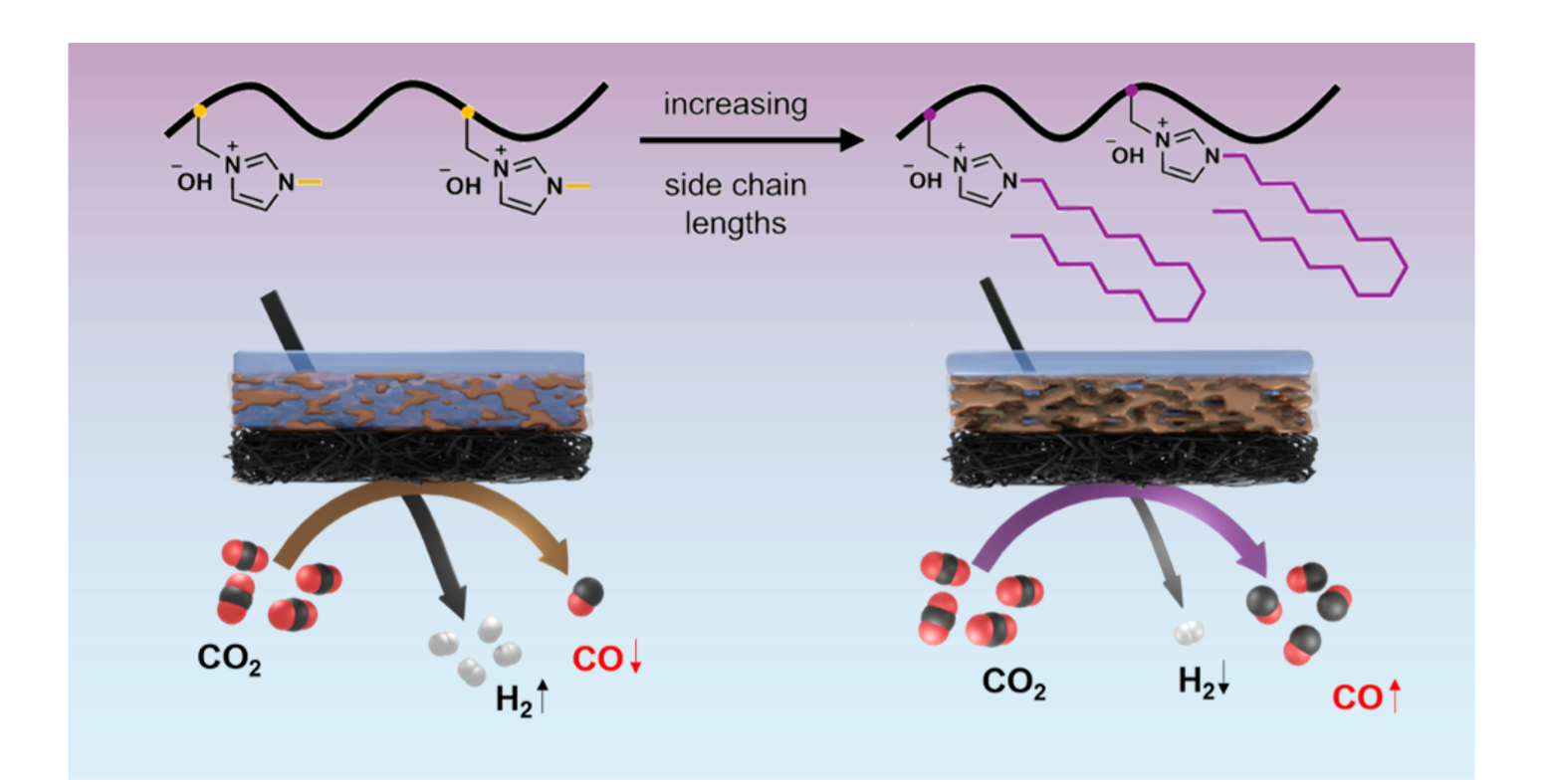
We report the structural engineering and application of 1-alkylimidazolium anion-exchange ionomers for Ag-catalyzed electrochemical CO2 reduction reaction (CO2RR) optimized toward syngas production. Systematic variation of alkyl side chain length (CnH2n+1 where n = 1, 4, 10, 16) and molecular weight (7, 20, 42 kg mol–1) reveals that specific ionomer designs can effectively modulate interfacial reaction environments and sustain conditions that promote CO2RR. Changes in ionomer architecture alter hydrophobicity and ionic conductivity, both of which strongly influence catalytic activity and product distributions. The ionomer with the longest n-hexadecyl side chains achieved CO selectivity of 79.3% and suppressed the hydrogen evolution reaction (HER) to 3.6%, compared to the methyl-substituted analog that favored HER. The n-hexadecyl ionomer also increased formate (HCOO–) selectivity to 17.9%, an unusually high value for Ag catalysts that reflects the elevated local pH at the interface. These results indicate that the ionomer creates microenvironments with both high hydrophobicity and elevated local pH, conditions favorable for CO2RR. Performance remained stable across varying bulk electrolyte conditions including dilute and acidic environments. In membrane electrode assemblies (MEAs) employing cation-exchange membranes, the optimized ionomer achieved CO selectivity of 68.7% at 3.0 V, representing an increase of 24.7% over the commercial Sustainion ionomer. These results underscore the critical role of ionomer design in shaping interfacial properties and guiding reaction pathways in electrochemical systems. This work positions rational ionomer design as a powerful strategy for advancing selective and efficient CO2 electrolysis.