2025 AIChE Annual Meeting
(487c) Shaping Stem Cell Fate: How Fluid Viscosity Drives Osteogenic and Immunomodulatory Priming of Human Mesenchymal Stem Cells
Authors
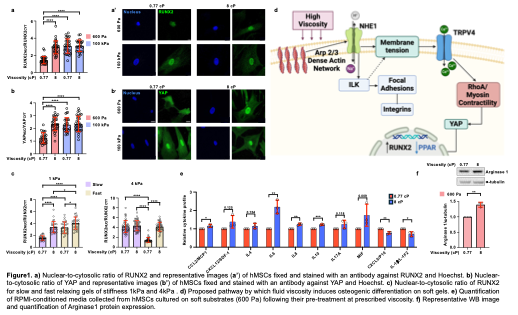
Using methylcellulose to elevate medium viscosity to 8cP - a level comparable to native bone marrow and synovial fluid - we observed that hMSCs cultured on otherwise adipogenic-inducing soft substrates (600 Pa) undergo a notable lineage switch toward osteogenesis. We characterized these changes via multiple osteogenic markers and techniques. This osteogenic bias overrides the instructive cues typically attributed to substrate stiffness(Fig.1a,b) and stress-relaxation dynamics (Fig.1c). Mechanistically, elevated viscosity activates a hierarchical mechano-transduction pathway beginning with Arp2/3 complex-mediated actin polymerization, which increases cortical actin density leading to membrane tension via enhanced Na⁺/H⁺ exchanger 1 (NHE1) activity and subsequently triggers TRPV4-mediated calcium influx. The integration of these signals activates the RhoA/ROCK pathway, leading to nuclear translocation of YAP and upregulation of the osteogenic transcription factor RUNX2 (Fig.1d).
Interestingly, 6 days exposure to high-viscosity conditions not only initiates osteogenic programming but also establishes a persistent cellular "memory" that maintains osteogenic phenotype post-withdrawal. Moreover, viscosity-primed hMSCs exhibit a robust immunosuppressive phenotype, characterized by increased secretion of IL-10 and IL-6 (Fig.1e). These secreted factors in the culture medium reprogram macrophages toward an M2-like regenerative phenotype, marked by a 1.5-fold increase in Arginase-1 expression (Fig.1f). This dual functionality—simultaneous osteogenic and immunomodulatory priming—offers a powerful strategy for enhancing therapeutic outcomes.
In conclusion, our findings introduce extracellular viscosity as a potent and tunable design parameter for stem cell-based regenerative medicine. The ability to non-genetically prime hMSCs in a short period offers a scalable, growth factor-free method to enhance therapeutic outcomes in bone regeneration and allogeneic transplantation. This strategy is particularly relevant for developing off-the-shelf stem cell therapies and bioengineered tissues with spatially graded mechanical properties.
References
Alice Amitrano et al., Extracellular fluid viscosity regulates human mesenchymal stem cell lineage and function. Science Advances 11, eadr5023 (2025). DOI:10.1126/sciadv.adr5023