2025 AIChE Annual Meeting
(575c) Separating Carbon Monoxide Using Electrochemistry
Authors
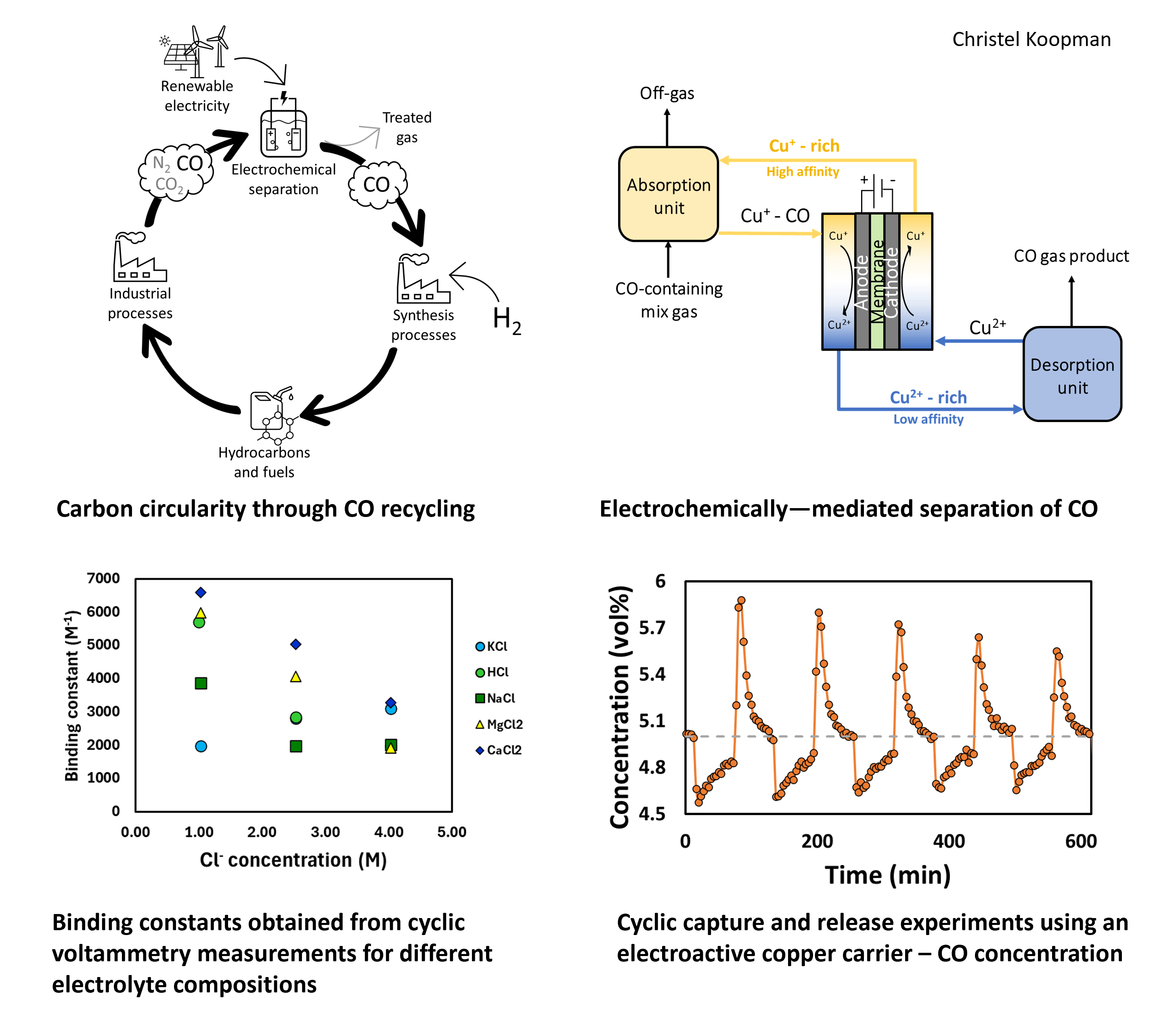
We aim to develop an electrochemically driven separation process for carbon monoxide. Carbon monoxide is both an important feedstock for the production of higher-value chemicals and fuels, as well as a by-product of certain industrial processes. Conventional separation methods fail to efficiently separate carbon monoxide from industrial off-gases, especially from N2, resulting in the CO being flared and emitted as CO2. It is estimated that recycling 77% of CO from the European steel industry can provide (and exceed) the feedstock requirement for Europe's methanol and ethanol production.[2] As part of the transition to a circular carbon economy, we aim to enable the recycling of CO from off-gases by developing an electrochemical separation process for carbon monoxide.
Taking inspiration from hemoglobin, we propose to leverage the affinity of transition metals, such as Fe2+, to carbon monoxide. The process relies on an electro-active sorbent material, where the affinity of carbon monoxide to this sorbent material depends on the oxidation state of the material and thus allows for capture and release by potential control.
The binding affinity of different electro-active carriers to CO is investigated using cyclic voltammetry measurements. We identified a couple of Cu-, Fe- and Ni-based complexes as suitable candidates for electrochemical separation of CO. The simplicity of the Cu-based carrier, made us further explore this material for electrochemical CO separation. The effect of electrolyte composition was studied, using different cation types and Cl- concentrations. The Cl- ions are essential for stabilizing Cu+ - which is the activated carrier - but the CV method showed that increasing Cl- concentrations decreases the binding affinity of the carrier. This trend is supported by DFT calculations and can be attributed to geometrical effects and electrostatic interactions between CO and Cu+. For operation, it will be important to find an optimal electrolyte composition that balances the stabilization of the carrier with carrier affinity.
We demonstrate the electrochemical separation of carbon monoxide from a mixed gas stream with 5 vol% of CO in nitrogen using the electro-active carriers. Capture and release experiments are performed with reduction and oxidation cycles in either H-cell or flow-cell configuration. Our study demonstrates that carbon monoxide can be selectively separated, and includes process optimization to improve the electrochemical separation performance such as faradaic efficiency, capacity utilization, and product recovery. The process optimization includes carrier concentration, operation potentials, liquid and gas flow rates, and electrochemical and sorption unit configuration. The study revealed that moving to a flow-cell configuration and introducing membrane contactors can increase the initial capture rate by a factor of 7 – improving product recovery. A higher oxidation potential can increase the rate of release but is accompanied by a decrease in faradaic efficiency. With our study, we highlight the potential and challenges of separating carbon monoxide from gas mixtures via an electrochemically-mediated separation process.
References
[1] Sholl, D.S. Lively, R.P. Nature 2016, 532, 435-437