2025 AIChE Annual Meeting
(118e) Self-Pillared Hierarchical Silicalite-1 Zeolites for Enhanced Suzuki-Miyaura Coupling Reactions
Author
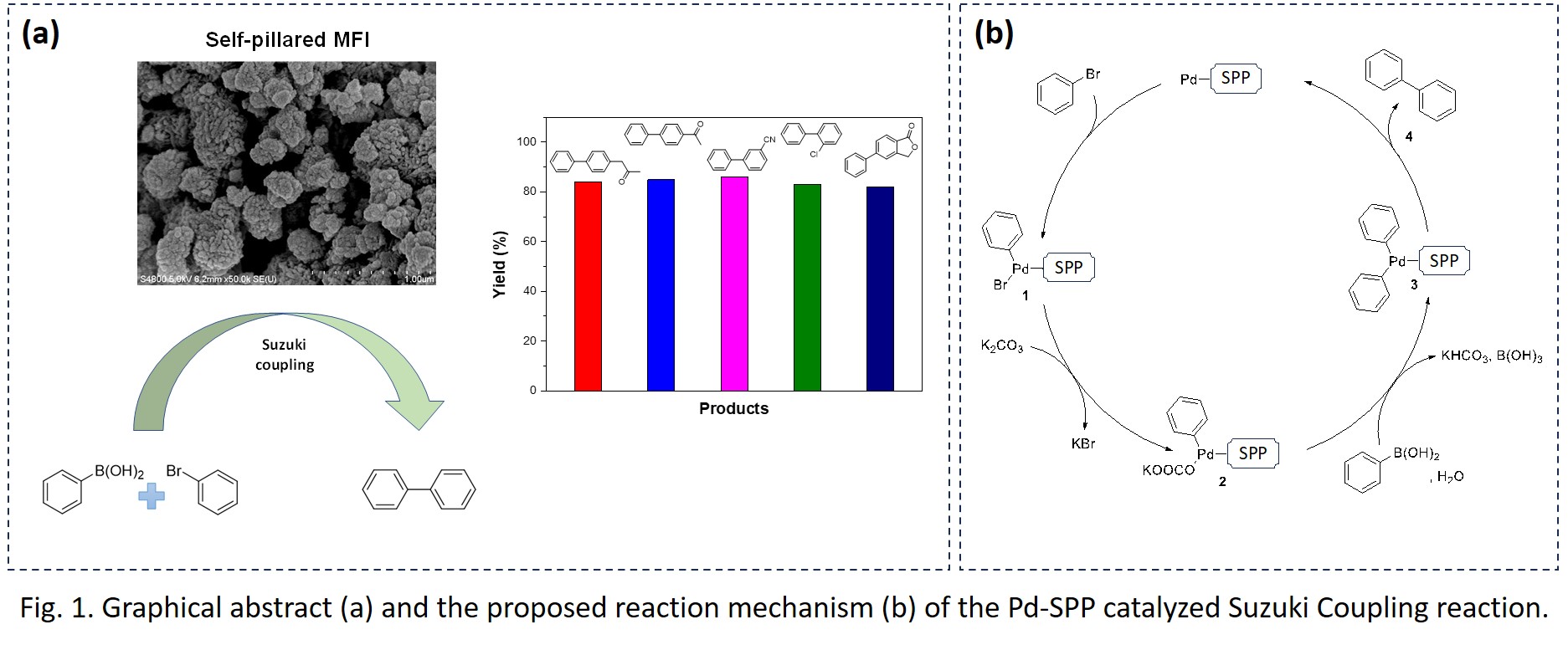
The Suzuki coupling reaction is a widely employed technique for the synthesis of biaryl compounds in various disciplines. This study introduces the development of a highly efficient and recyclable palladium-doped Silicalite-1 (S-1) catalyst featuring a hierarchical structure, which enhances the efficacy of Suzuki coupling reactions. By utilizing tetra-n-butylphosphonium hydroxide as a structure-directing agent and adjusting the molar ratios of ethanol and water in the synthetic precursor, we successfully produced a range of porous S-1 catalysts. These catalysts displayed a unique architecture characterized by interconnected thin pillars or lamellae. The catalyst's remarkable specific activity facilitated rapid Suzuki coupling reactions, completing within just three hours under environmentally benign conditions. The Suzuki reaction mechanism was discussed, which involves an oxidative addition of bromobenzene to heterogeneous Pd, followed by metal exchange with phenyl boronic acid and completed by a reductive elimination. Comprehensive substrate screening, selectivity assessments, and recycling studies were also undertaken.