2025 AIChE Annual Meeting
(164c) Selective Ion Enrichment through Chelating Group-Functionalized Membranes
Authors
Fiona Chen - Presenter, Rice University
Njideka Nnorom, Rice University
Timauri-Lee Carby, Rice University
Rafael Verduzco, California Institute of Technology
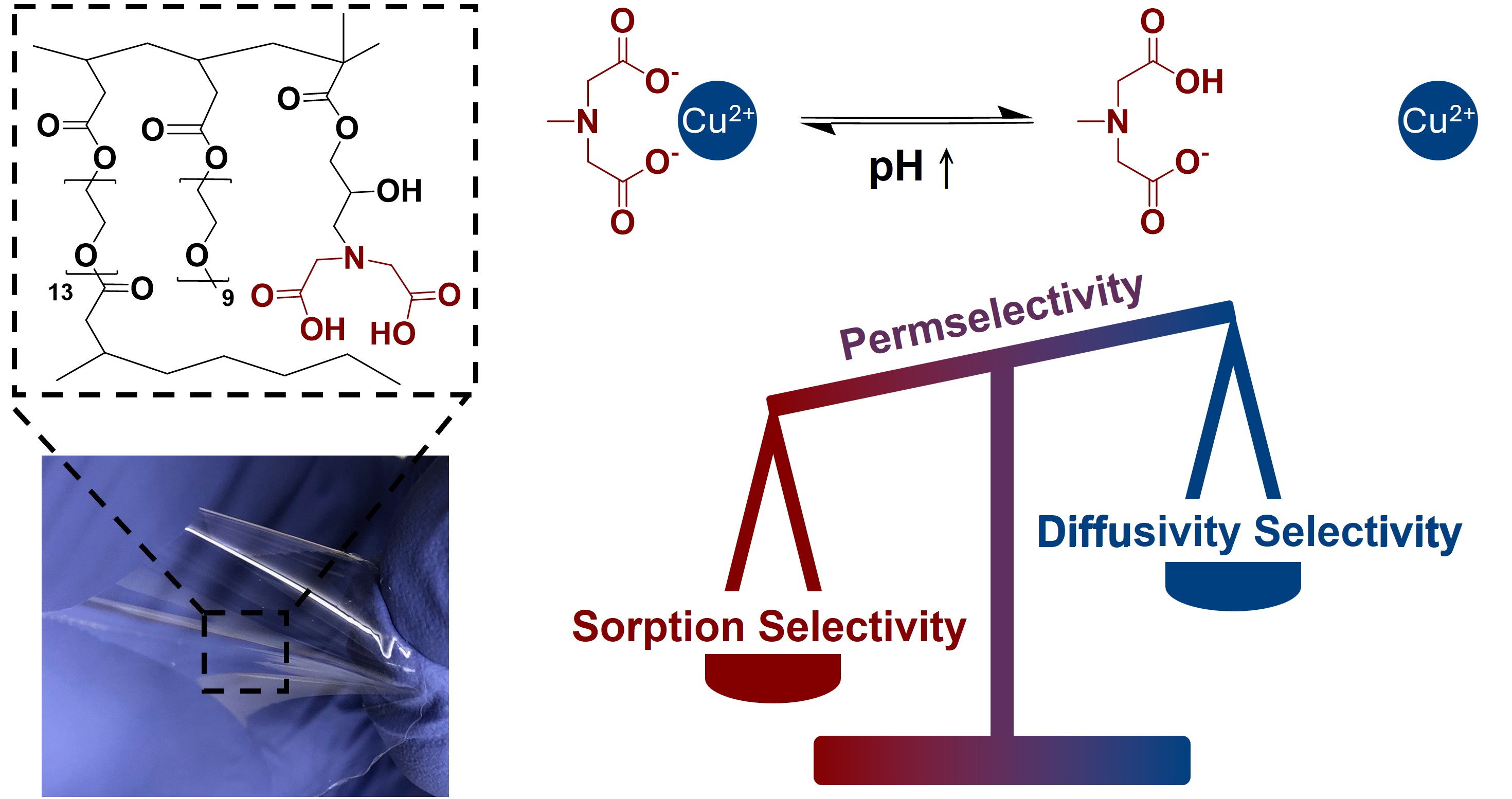
Cost-effective technologies for recovering or recycling materials from waste streams are needed to support the growing demand for critical elements and materials. In this work, we investigate ligand-functionalized membranes with ion-specific selectivity towards Cu2+ over Ni2+ and Mg2+. We prepared a series of membranes functionalized with the iminodiacetic acid (IDA) chelating group using both acrylate-based free radical reactions and layer-by-layer assembly. IDA binds preferentially to Cu2+ over Ni2+ and Mg2+. The acrylate-IDA membranes varied systematically in terms of IDA content and water content, and the salt sorption, diffusion, and permeation were quantified over a range of solution pH values, which impact the binding energy of the IDA group to divalent cations. At pH 1, the acrylate-based IDA-functionalized membranes exhibited strong sorption selectivity for CuCl2, which increased with increasing IDA content. However, the membranes showed preferential diffusion for NiCl2 and MgCl2 over CuCl2. Despite this, at pH 1, where sorption selectivity was highest, the membranes were perm-selective towards CuCl2 over NiCl2 and MgCl2. Furthermore, the permselectivity increased to a value of 2.8 for Cu2+ over Ni2+ with decreasing water content, revealing a permselectivity-permeability trade-off for ion-specific membranes, which has not been previously reported. On the other hand, the layer-by-layer assembly coating made using polyallylamine hydrochloride (PAH) and poly[(N,N-dicarboxymethyl)allylamine] (PDCMAA) exhibited properties of ultrathin, high functional group concentration, and high ionic flux. The transport properties in terms of mixed salt flux and permselectivity were evaluated for membranes with different coating thickness using diffusion cells, revealing permselectivity of up to 18 for Cu2+ over Mg2+ and 14 for Cu2+ over Ni2+ for coatings exceeding 6 bilayers (~150 nm thickness). In addition, we demonstrated that the membranes can be used in pressure-driven separation processes. This work provides insight into the design of membranes with ion-specific permeabilities and highlight key factors such as, ion-specific ligand content, water content, and solution pH, which influences sorption, diffusion, and permeability selectivity. These findings can serve as design strategies for the recovery of ions of interest and facilitate the development of ion-selective separations processes for recovering Cu2+ and other valuable ions.