2025 AIChE Annual Meeting
(584cu) Selective Hydrogenation with Ternary Intermetallic Catalysts
Authors
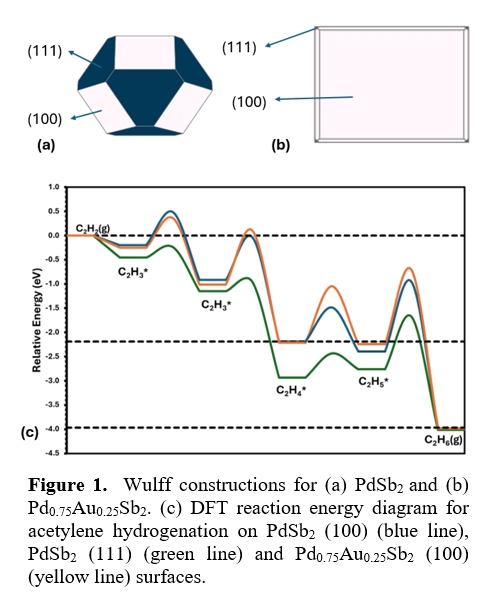
Using an integrated approach combining high-throughput design, density functional theory, microkinetic modeling, and experimental validation, we demonstrate that incorporating a third element enables fine-tuning of hydrogenation activity and selectivity. For example, adding Au to PdSb₂ alters the nanoparticle’s shape by suppressing the (111) facet and favoring the (100) surface. Although Au is not directly involved at the active site, it modulates which surface facets are exposed, which in turn affects the catalytic pathway. This prediction is supported by surface energy calculations and validated by electron backscatter diffraction.
Experimental reactivity measurements and modeling show that changes in facet exposure influence surface coverage and reaction energetics, thereby impacting the semi-hydrogenation of acetylene. Microkinetic simulations confirm that the modified surface structure leads to improved selectivity by altering key intermediates and pathways.
Ternary intermetallics expand the design space for catalyst development, enabling precise control of active site structure and reactivity. The computationally-guided approach demonstrates a powerful strategy for rationally designing selective hydrogenation catalysts.